
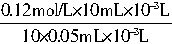 =2.4mol/L��
=2.4mol/L��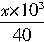 mol��������ζ�ϴ��Һʱ������1�����ᣬ��2m3��ϴ��Һ�м���һ���ռ�൱�ڽ�һ���ռ����Ƴ�2m3����Һ��ȡ��10mL����1�θ�����ǡ����ȫ��Ӧ��
mol��������ζ�ϴ��Һʱ������1�����ᣬ��2m3��ϴ��Һ�м���һ���ռ�൱�ڽ�һ���ռ����Ƴ�2m3����Һ��ȡ��10mL����1�θ�����ǡ����ȫ��Ӧ��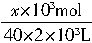 ��0.01L=0.05��10-3L��2.4mol/L��
��0.01L=0.05��10-3L��2.4mol/L��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com