
(10��)�Ҷ���(HOOC-COOH)�׳Ʋ��ᣬ����㷺�����ڶ���ֲ���ϸ��Ĥ�ڣ��侧��ͨ�����нᾧˮ(H2C2O4��2H2O)��������۵�Ϊ101.5�棬��ˮ������۵�Ϊ189.5�档����������������157��ʱ��������������ʼ�ֽ⣬�ֽ����ΪCO��CO2��H2O��
ij�о���ѧϰС���ͬѧ�����Բ���ķֽⷴӦ����̽������Ƴ�����ʵ����֤�Ҷ���ķֽⲢ�ⶨ��ֽ��ʣ������������£�
���Ȱ��Ҷ��ᾧ����ں����н��к濾��ȥ���ᾧˮ�����á�
�ڰ�ͼ2���Ӻ�װ�á�
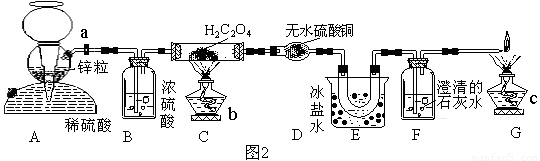
�ۼ��װ�õ������ԡ�
�ܴ���a��ͨ��H2һ������ٹر�a����Ȼ�ƾ���b��c��
�ݵ�Cװ���й�����ʧ��ֹͣ����
����a������ͨ��H2��ֱ����ȴ��
�Իش��������⣺
��װ��A������______________________________��B������_____________________��װ��E������_____________________________��
�Ƽ������װ�õ������Եķ�����____________________________________________��
�����ֽ��������ͨ����������ʹ������Ҷ���ķֽ���_________(���������С�����䡱)��
�ȿ��Դ���װ��G�ķ�����_____________________________________________��
������ȡH2C2O4(���)������Ϊ4.5g��ʵ������Ƶ�D��E��F�ֱ�����0.95g��0.40g��1.98g�����Ҷ���ķֽ���Ϊ_______________��
�Ų����������ų�װ���ڵ�CO2���ֽ����ɵ�CO2ȫ������F�У�����H2(���ȥ�����л��е�ˮ����)��ʹ�������Ҷ���������ȴΪ���壬�������ʵ��(4��)�������������м�������ˮ��Ȼ����˫�ֻ���ë����ס�������۲�Fƿ���������ݲ������������ݲ�����˵����©��������������(2��)���Ǽ�С(1��)����������(����)�ռ�����(1��)����90%(2��)��
����������A�п�ʼ������H2�ɽ�װ���ڵ�ˮ������CO2�ų���������Ϊʹ�Ҷ���ֽ����������ȫ��ͨ��D��E��Fװ�ã�����ֽ����������������װ��C�У���A�����������к���ˮ�����������ȥ���Ҷ���Ϊ�л�����Ȼ�ӷ�������������ͨ��E�DZ���ȴΪ���壬�Ա���Ժ���ʵ��������š������������м�������ˮ��Ȼ����˫�ֻ���ë����ס�������۲�Fƿ���������ݲ������Dz���ͨ�����������Ҷ���ֽ������CO2���в�������C��F֮��������У����õ��ķֽ��ʼ��١�����������õ�����ΪCO���ж�������������У�����ȼ���⣬����������(����)�ռ�����������H2C2O4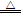 CO2��+CO��+H2O������F����1.98g�������ɵ�CO2����Ϊ1.98g���ɵó��ֽ��H2C2O4Ϊ4.05g�����Ҷ���ķֽ���Ϊ90%����������У����ܲ���D��Eװ�õ��������ݣ���δ�ֽ���Ҷ�������Dװ���оͻ��в�������ȴ�����̣�Ҳ����˵0.95g����ȫ������ˮ��������0.40g������ȫ��δ�ֽ���Ҷ�����������ɼ��������������ݸ��ţ����������ھ��������������⼫���´���
CO2��+CO��+H2O������F����1.98g�������ɵ�CO2����Ϊ1.98g���ɵó��ֽ��H2C2O4Ϊ4.05g�����Ҷ���ķֽ���Ϊ90%����������У����ܲ���D��Eװ�õ��������ݣ���δ�ֽ���Ҷ�������Dװ���оͻ��в�������ȴ�����̣�Ҳ����˵0.95g����ȫ������ˮ��������0.40g������ȫ��δ�ֽ���Ҷ�����������ɼ��������������ݸ��ţ����������ھ��������������⼫���´���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.��98����Ũ��������100 g 10����ϡ������Һʱ����ʹ����Ͳ����õ�����ƿ
B.����������������л��е�NaNO3����
C.���÷�Һ©�����Ҷ�����ˮ�Ļ��Һ�����
D.��Ũ��ˮϴ������������Ӧ���Թ�
E.����Ũ�����Ũ����Ļ����ʱ����Ũ�����������������뵽Ũ�����У������Ͻ���
F.����ʽ�ζ�����ȡ20.00mL0.100 0 mol/L��KMnO4��Һ
G.��������ƽ��ȡ10.50g�����NaCI����
H.����ѧ������ͭ������ᾧˮ�����ⶨ����ʵ���У���������������Ҫ�Ĵ�
I.������������NaOH��Na2CO3��������
J.����������Һʱ����ϡ��ˮ�����μӵ���������Һ�У���������������μӵ�
�����պ��ܽ�Ϊֹ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ�ʵ����
��4�֣������й�ʵ�����������ȷ���ǣ�����ţ�
A����98%��Ũ��������100g10%��ϡ������Һʱ����ʹ����Ͳ����õ�����ƿ
B������������������л��е�NaNO3����
C�����÷�Һ©�����Ҷ�����ˮ�Ļ��Һ�����
D����Ũ��ˮϴ������������Ӧ���Թ�
E������Ũ�����Ũ����Ļ����ʱ����Ũ�����������������뵽Ũ�����У������Ͻ���
F������ʽ�ζ�����ȡ20.00mL 0.1000mol/L��KMnO4��Һ
G����������ƽ��ȡ10.50g�����NaCl����
H������ѧ������ͭ������ᾧˮ�����ⶨ����ʵ���У���������������Ҫ�Ĵ�
I��������������NaOH��Na2CO3��������
J������������Һʱ����ϡ��ˮ�����μӵ���������Һ�У���������������μӵ������պ��ܽ�Ϊֹ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��4�֣������й�ʵ�����������ȷ���ǣ�����ţ�
A����98%��Ũ��������100g10%��ϡ������Һʱ����ʹ����Ͳ����õ�����ƿ
B������������������л��е�NaNO3����
C�����÷�Һ©�����Ҷ�����ˮ�Ļ��Һ�����
D����Ũ��ˮϴ������������Ӧ���Թ�
E������Ũ�����Ũ����Ļ����ʱ����Ũ�����������������뵽Ũ�����У������Ͻ���
F������ʽ�ζ�����ȡ20.00mL 0.1000mol/L��KMnO4��Һ
G����������ƽ��ȡ10.50g�����NaCl����
H������ѧ������ͭ������ᾧˮ�����ⶨ����ʵ���У���������������Ҫ�Ĵ�
I��������������NaOH��Na2CO3��������
J������������Һʱ����ϡ��ˮ�����μӵ���������Һ�У���������������μӵ������պ��ܽ�Ϊֹ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ��ʦ���и�����ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ�ʵ����
��4�֣������й�ʵ�����������ȷ���ǣ�����ţ�
| A����98%��Ũ��������100g10%��ϡ������Һʱ����ʹ����Ͳ����õ�����ƿ |
| B������������������л��е�NaNO3���� |
| C�����÷�Һ©�����Ҷ�����ˮ�Ļ��Һ����� |
| D����Ũ��ˮϴ������������Ӧ���Թ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ����ʦ���и�����һ���¿���ѧ�Ծ� ���ͣ�ʵ����
��4�֣������й�ʵ�����������ȷ���ǣ�����ţ�
| A����98%��Ũ��������100g10%��ϡ������Һʱ����ʹ����Ͳ����õ�����ƿ |
| B������������������л��е�NaNO3���� |
| C�����÷�Һ©�����Ҷ�����ˮ�Ļ��Һ����� |
| D����Ũ��ˮϴ������������Ӧ���Թ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com