
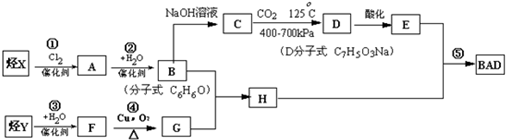
���÷�����X������Y���Ժϳ����������ռ�BAD����֪G���ܷ���������Ӧ��BAD
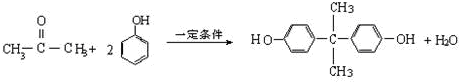
�ṹ��ʽΪ��
BAD�ĺϳ�·�����£�
�Իش�
(1) д���ṹ��ʽY________________________ D____________________��
(2) ����ȡ����Ӧ����_____________________________����������ţ� ��
(3) 1molBAD�����뺬____molNaOH����Һ��ȫ��Ӧ
(4) д����Ӧ�ܷ���ʽ________________________________________________
�� д��B+G��H����ʽ________________________________________________��
(6) E��ͬ���칹�����������ʣ�����FeCl3��Ӧ����Һ����ɫ�������ܷ���������Ӧ�����ͬ���칹�干�� ��
 ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�





�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�










�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�








�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�







�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���÷�����X������Y���Ժϳ����������ռ�BAD����֪G���ܷ���������Ӧ��BAD�ṹ��ʽΪ��
OHCOOCCH3CH3OOCOH
BAD�ĺϳ�·�����£�

�Իش��������⣺
(1)д���������ʵĽṹ��ʽ��Y�� ����D ��
H�� ����
(2)����ȡ����Ӧ�������٢ڢ���(���������)��
(3)1mol BAD�����뺬���� mol NaOH����Һ��ȫ��Ӧ��
(4)д����Ӧ�ܵĻ�ѧ����ʽ�� ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com