
·ÖĪö ijČÜŅŗÖŠæÉÄÜŗ¬ÓŠNa+”¢NH4+”¢Cl-”¢SO42-”¢NO3-”¢CO32-£¬
£Ø1£©Č”Ņ»¶ØĮæµÄČÜŅŗÓŚŹŌ¹ÜÖŠ£¬¼ÓČė¹żĮæµÄBa£ØNO3£©2ČÜŅŗÓŠ°×É«³ĮµķÉś³É£¬¹żĀĖ³öµÄ³Įµķ²æ·ÖČÜÓŚĻ”HNO3£¬ĖµĆ÷ŌČÜŅŗÖŠ¼“ÓŠSO42-»¹ÓŠCO32-£¬ĮņĖį±µ²»ČÜÓŚĻ”ĻõĖį£¬Ģ¼Ėį±µČÜÓŚĻ”ĻõĖį£¬²śÉś¶žŃõ»ÆĢ¼ĘųĢå5.6L£Ø±źæöĻĀ£©£¬ĪļÖŹµÄĮæĪŖ0.25mol£¬øł¾ŻŌ×ÓŹŲŗć£¬ŌČÜŅŗÖŠµÄCO32-µÄĪļÖŹµÄĮæĪŖ0.25mol£»Ź£Óą³ĮµķŹĒĮņĖį±µ£¬ĘäÖŹĮæ4.66g£¬ĮņĖį±µµÄĦ¶ūÖŹĮæŹĒ233g/mol£¬ŌņĘäĪļÖŹµÄĮæĪŖ0.02mol£¬Ķ¬Ąķøł¾ŻŌ×ÓŹŲŗćæÉÖŖŌČÜŅŗÖŠµÄSO42-µÄĪļÖŹµÄĮæĪŖ0.02mol£»
£Ø2£©Č”²æ·ÖĀĖŅŗ£¬¼ÓČė¹żĮæµÄNaOHČÜŅŗ²¢¼ÓČČ£¬²śÉś13.6gĪŽÉ«ÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄĘųĢ壬øĆĘųĢåÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£¬²śÉśµÄĘųĢåŹĒ°±Ęų£¬13.6g°±ĘųµÄĪļÖŹµÄĮæĪŖ0.8mol£¬øł¾ŻŌ×ÓŹŲŗćæÉÖŖŌČÜŅŗÖŠNH4+µÄĪļÖŹµÄĮæĪŖ0.8mol£»
£Ø3£©ĮķČ”²æ·ÖĀĖŅŗ£¬¼ÓČėAgNO3ČÜŅŗ£¬ĪŽĆ÷ĻŌĻÖĻó£¬Ö¤Ć÷ŌČÜŅŗÖŠĪŽĀČĄė×Ó£®
½ā“š ½ā£ŗ¾ŻÉĻĆę·ÖĪöµĆ³öŌČÜŅŗÖŠŅ»¶ØÓŠµÄĄė×ÓŹĒ£ŗNH4+”¢SO42-”¢CO32-£¬Ņ»¶Ø²»ŗ¬Cl-£¬ČÜŅŗÖŠNH4+ÓŠ0.8mol£¬ŌņŃōĄė×ÓĖł“ųµēŗÉÓŠ0.8mol£¬SO42-ÓŠ0.02mol£¬CO32-ÓŠ0.25mol£¬ŌņŅõĄė×ÓĖł“ųøŗµēŗÉÓŠ0.04mol+0.5mol=0.54mol£¬øł¾ŻµēŗÉŹŲŗćĖµĆ÷ČÜŅŗÖŠ±ŲŠė»¹ÓŠĘäĖūŅõĄė×Ó£¬ĖłŅŌNO3-Ņ»¶ØÓŠ£¬¶ųNa+æÉÄÜÓŠ£¬
¢ŁČÜŅŗÖŠæĻ¶Ø“ęŌŚµÄĄė×ÓŹĒCO32-”¢SO42-”¢NH4+”¢NO3-£¬
¹Ź“š°øĪŖ£ŗCO32-”¢SO42-”¢NH4+”¢NO3-£»
¢ŚČÜŅŗÖŠæĻ¶Ø²»“ęŌŚµÄĄė×ÓŹĒCl-£¬
¹Ź“š°øĪŖ£ŗCl-£»
¢ŪČÜŅŗÖŠæÉÄÜ“ęŌŚµÄĄė×ÓŹĒNa+£¬
¹Ź“š°øĪŖ£ŗNa+£»
¢ÜŹµŃé£Ø1£©ÖŠ³Įµķ²æ·ÖŹĒĢ¼Ėį±µČÜÓŚĻ”HNO3²¢·Å³öĪŽÉ«ĪŽĪ¶ĘųĢåµÄĄė×Ó·½³ĢŹ½£ŗBaCO3+2H+=Ba2++H2O+CO2”ü£¬
¹Ź“š°øĪŖ£ŗBaCO3+2H+=Ba2++H2O+CO2”ü£»
¢ŻŹµŃé£Ø2£©ÖŠ²śÉśĘųĢåµÄĄė×Ó·½³ĢŹ½ĪŖ£ŗNH4++OH-$\frac{\underline{\;\;”÷\;\;}}{\;}$NH3”ü+H2O£¬
¹Ź“š°øĪŖ£ŗNH4++OH-$\frac{\underline{\;\;”÷\;\;}}{\;}$NH3”ü+H2O£®
µćĘĄ ±¾Ģā漲鳣¼ūĄė×ӵļģŃ飬³ż¶ØŠŌŹµŃéµÄÅŠ¶ĻĶā£¬»¹ÓŠ¶ØĮæŹµŃéµÄŹż¾Ż·ÖĪö£¬×¢ŅāµēŗÉŹŲŗćµÄŌĖÓĆ£¬ŹōÓŚŅדķĢā£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£®ÄæµÄ£ŗÅØ¶Č¶Ō»Æѧ·“Ó¦ĖŁĀŹµÄÓ°Ļģ | B£®ÄæµÄ£ŗÅäÖĘŅų°±ČÜŅŗ |
¼ÓČė 1mol/L 1mLĮņĖį””””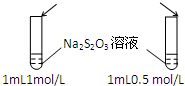 | 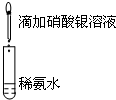 ”””” ”””” |
| C£®ÄæµÄ£ŗ±Č½ĻAl”¢Fe”¢Cu»ī¶ÆŠŌ | D£®ÄæµÄ£ŗŹµŃéŹŅÖĘČ”°±Ęų |
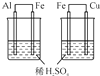 |  |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
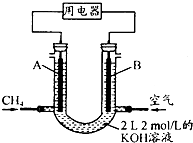 ¾Ż±ØµĄ£¬ŌŚĪ÷²Ų¶³ĶĮµÄŅ»¶ØÉī¶ČĻĀ£¬·¢ĻÖĮĖ“¢Į澎“óµÄ”°æÉČ¼±ł”±£¬ĖüÖ÷ŅŖŹĒ¼×ĶéŗĶĖ®ŠĪ³ÉµÄĖ®ŗĻĪļ£ØCH4•nH2O£©£®
¾Ż±ØµĄ£¬ŌŚĪ÷²Ų¶³ĶĮµÄŅ»¶ØÉī¶ČĻĀ£¬·¢ĻÖĮĖ“¢Į澎“óµÄ”°æÉČ¼±ł”±£¬ĖüÖ÷ŅŖŹĒ¼×ĶéŗĶĖ®ŠĪ³ÉµÄĖ®ŗĻĪļ£ØCH4•nH2O£©£®| »Æѧ¼ü | H-H | C-O | C”ŌO | H-O | C-H |
| E/£ØkJ•mol-1£© | 436 | 343 | 1 076 | 465 | 413 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | K | B£® | Ca | C£® | I | D£® | Ne |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā

| A£® | Č”“ś·“Ó¦ | B£® | ¼Ó³É·“Ó¦ | C£® | ¾ŪŗĻ·“Ó¦ | D£® | ¼Ó¾Ū·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | °±Ņ×Ņŗ»Æ£¬Ņņ“ĖæÉÓĆĄ“×÷ÖĘĄä¼Į | B£® | °±Ė®ĻŌČõ¼īŠŌ£¬ÄÜŹ¹·ÓĢŖČÜŅŗ±äŗģ | ||
| C£® | °±¼«Ņ×ČÜÓŚĖ®£¬Ņņ“Ė°±Ė®±Č½ĻĪČ¶Ø | D£® | °±¼«Ņ×ČÜÓŚĖ®£¬Ņņ“ĖæÉ×÷ÅēČŖŹµŃé |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
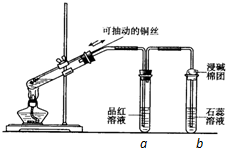 ijĶ¬Ń§Éč¼ĘŹµŃéÖ¤Ć÷ĶÓėÅØĮņĖįÄÜ·¢Éś·“Ó¦£¬²¢¼ģŃéÉś³ÉĘųĢåµÄŠŌÖŹ£¬ČēĶ¼ĖłŹ¾£¬ŌŚŹŌ¹ÜĄļ¼ÓČė2mLÅØĮņĖį£¬ÓĆ“ųµ¼¹ÜŗĶŅ»øöŠ”æ׵Ľŗ¹ÜČū½ō£¬“Óæ×ÖŠ²åČėŅ»øłĶĖ棬¼ÓČČ£¬°Ń·Å³öµÄĘųĢåŅĄ“ĪĶØČėĘ·ŗģČÜŅŗŗĶŹÆČļČÜŅŗÖŠ£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
ijĶ¬Ń§Éč¼ĘŹµŃéÖ¤Ć÷ĶÓėÅØĮņĖįÄÜ·¢Éś·“Ó¦£¬²¢¼ģŃéÉś³ÉĘųĢåµÄŠŌÖŹ£¬ČēĶ¼ĖłŹ¾£¬ŌŚŹŌ¹ÜĄļ¼ÓČė2mLÅØĮņĖį£¬ÓĆ“ųµ¼¹ÜŗĶŅ»øöŠ”æ׵Ľŗ¹ÜČū½ō£¬“Óæ×ÖŠ²åČėŅ»øłĶĖ棬¼ÓČČ£¬°Ń·Å³öµÄĘųĢåŅĄ“ĪĶØČėĘ·ŗģČÜŅŗŗĶŹÆČļČÜŅŗÖŠ£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NaOH£Øaq£©+$\frac{1}{2}$H2SO4£Øaq£©ØT$\frac{1}{2}$Na2SO4£Øaq£©+H2O£Øl£©£»”÷H1 | |
| B£® | NaOH£Øaq£©+$\frac{1}{2}$H2SO4£ØÅØ£©ØT$\frac{1}{2}$Na2SO4£Øaq£©+H2O£Øl£©£»”÷H4 | |
| C£® | NaOH£Øaq£©+HCl£Øaq£©ØTNaCl£Øaq £©+H2O£Øl£©£»”÷H2 | |
| D£® | CH3COOH£Øaq£©+NaOH£Øaq£©ØTCH3COONa £Øaq £©+H2O£Øl£©£»”÷H3 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com