
���� ���ڵ�һ����Һ�м�������ϡ���ᣬ�������ִ����ĺ���ɫ���壬˵������NO2����Ӧ����NO2-������Һ�в�����Fe2+��MnO4-�����ӣ�
���ڵڶ�����Һ�м�������������������Һ����ּ��Ⱥ��ռ���2.24L���壨��״���£���Ӧ���ɰ�����˵������NH4+����Ϊ0.1mol���������õ���Һ��ͨ������Ķ�����̼�����յõ�7.8g��ɫ������ӦΪAl��OH��3������Ϊ0.1mol��˵������Al3+����һ��������CO32-�����ӣ�
���ڵ�������Һ�м����������Ȼ�����Һ�����յõ�46.6g��ɫ������ӦΪBaSO4��Ϊ0.2mol��˵������SO42-������Ba2+��
����3n��Al3+��+n��NH4+��=2n��SO42-������3��0.1mol+0.2mol=2��0.2mol���������NO2-�����ɵ���غ��֪һ������K+���Դ˽����⣮
��� �⣺���ڵ�һ����Һ�м�������ϡ���ᣬ�������ִ����ĺ���ɫ���壬˵������NO2����Ӧ����NO2-������Һ�в�����Fe2+��MnO4-�����ӣ�
���ڵڶ�����Һ�м�������������������Һ����ּ��Ⱥ��ռ���2.24L���壨��״���£���Ӧ���ɰ�����˵������NH4+����Ϊ0.1mol���������õ���Һ��ͨ������Ķ�����̼�����յõ�7.8g��ɫ������ӦΪAl��OH��3������Ϊ0.1mol��˵������Al3+����һ��������CO32-�����ӣ�
���ڵ�������Һ�м����������Ȼ�����Һ�����յõ�46.6g��ɫ������ӦΪBaSO4��Ϊ0.2mol��˵������SO42-������Ba2+��
����3n��Al3+��+n��NH4+��=2n��SO42-������3��0.1mol+0.2mol=2��0.2mol���������NO2-�����ɵ���غ��֪һ������K+��
��1������NO2-���������ᷢ��2H++2NO2-�TNO��+NO2��+H2O���ʴ�Ϊ��2H++2NO2-�TNO��+NO2��+H2O��
��2��ʵ����в�����ɫ����ΪAl��OH��3����Ӧ�����ӷ���ʽΪAlO2-+CO2+2H2O=Al��OH��3��+HCO3-���ʴ�Ϊ��AlO2-+CO2+2H2O=Al��OH��3��+HCO3-��
��3�������Ϸ�����֪���϶����ڵ�������NO2-��Al3+��SO42-��K+��NH4+���ʴ�Ϊ��NO2-��Al3+��SO42-��K+��NH4+��
��4������ȷ��NO2-����������ȷ���Ƿ���Cl-����ȡ����ԭ��Һ���Թ��У��μ����������ᱵ��Һ�����ˣ�������Һ�еμ������ữ����������Һ�������ɳ�����˵������Cl-�����������ɣ�˵������Cl-��
�ʴ�Ϊ��Cl-����ȡ����ԭ��Һ���Թ��У��μ����������ᱵ��Һ�����ˣ�������Һ�еμ������ữ����������Һ�������ɳ�����˵������Cl-�����������ɣ�˵������Cl-��
���� ���⿼���˳������ӵļ��顢���ӹ�����жϣ���Ŀ�Ѷ��еȣ�ע�����ճ������ӵĻ�ѧ���ʼ����鷽�����ܹ����ݵ���غ�ƽ����Һ��δ֪���ӵĴ����������ȷ���������Ϣ�ǽ����Ĺؼ����״���Ϊ�����ӵ��жϣ�����ʱע�⣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Fe2O3��ϡ���ᷴӦ | B�� | Al��OH��3��ϡ���ᷴӦ | ||
| C�� | CuO��ϡ���ᷴӦ | D�� | Fe��OH��2��ϡ���ᷴӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڿ����У�������Ʒ������Ʒ��������������ʴ | |
| B�� | ����ϩ�������ϻ�������Ϊ�����˼ӳɷ�Ӧ | |
| C�� | ʳƷ��װ���г�����С������ʯ�ң�Ŀ���Ƿ�ֹʳƷ�������� | |
| D�� | ��������ָ������һ��ָ������ΪPM2.5��ָ���ǿ�����ֱ����2.5�Ĺ����Һ����ܳ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
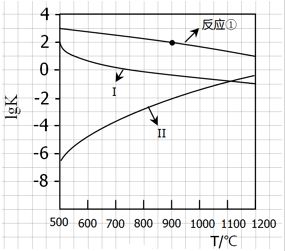 ��CaSO4����O2��ȼ��CO��Ӧ���ȿ������ȼ��Ч�ʣ����ܵõ��ߴ�CO2����һ�ָ�Ч����ࡢ���õ�����ȼ�ռ�������Ӧ��Ϊ����Ӧ����Ӧ�ں͢�Ϊ����Ӧ��
��CaSO4����O2��ȼ��CO��Ӧ���ȿ������ȼ��Ч�ʣ����ܵõ��ߴ�CO2����һ�ָ�Ч����ࡢ���õ�����ȼ�ռ�������Ӧ��Ϊ����Ӧ����Ӧ�ں͢�Ϊ����Ӧ��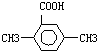 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
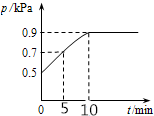 ��10L�ܱ������з���0.50 mol X����һ���¶��·�����Ӧ��X��g��?2Y��g��+Z��s����H��0��������������ѹǿp�淴Ӧʱ��t�ı仯��ϵ��ͼ��ʾ�����·�����ȷ���ǣ�������
��10L�ܱ������з���0.50 mol X����һ���¶��·�����Ӧ��X��g��?2Y��g��+Z��s����H��0��������������ѹǿp�淴Ӧʱ��t�ı仯��ϵ��ͼ��ʾ�����·�����ȷ���ǣ�������| A�� | ���¶��´˷�Ӧ��ƽ�ⳣ��K=0.64mol/L | |
| B�� | �ӷ�Ӧ��ʼ��t1ʱ��ƽ����Ӧ����v��X��=0.008mol/��L•min�� | |
| C�� | �����ƽ����ϵ��Y�������������������ϵ�¶Ȼ����Z���� | |
| D�� | �����������䣬�ٳ���0.1 mol ����X��ƽ�������ƶ���X��ת�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
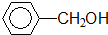 ����-OH
����-OH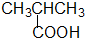 ������-COOH
������-COOH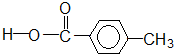 ȩ��
ȩ��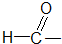
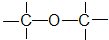
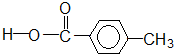 ����
����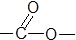
| A�� | �٢ڢۢܢ� | B�� | �ڢ� | C�� | �ڢۢ� | D�� | �ڢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ᷴӦ�����Ͻ������ᷴӦ | |
| B�� | ������Ӳ�Ⱥ�ǿ�Ⱦ��������Ͻ� | |
| C�� | ����������Ͻ�ֻ������Ԫ�� | |
| D�� | һ�������£����ۿ���ˮ������Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com