
X��Y��Z��Q��MΪ�����Ķ�����Ԫ�أ���ԭ���������������й���Ϣ���±�:
| X | ��ֲ����������ȱ�ٵ�Ԫ�أ��ǵ����ʵ���Ҫ�ɷ� |
| Y | �ؿ��к����ӵ�һλ |
| Z | ����������ԭ�Ӱ뾶��� |
| Q | �����д���ʹ����Ͻ���Ʒ����ҵ�Ͽ��õ����������ķ����Ʊ� |
| M | ��ˮ�д���������Ԫ��֮һ������������ϼ��븺�۵Ĵ�����Ϊ6 |
����8�֣�
��1�� 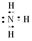
��2��A C D���д����÷֣����2����1�֣�ȫ�Ե�2�֣�
��3��3C��s��+ Al 2O3��s��= 2Al��s��+3CO��g�� ��H=" -(2a-2b)" kJ/mol �� ��H=" 2b" -2a kJ/mol
��4��8NO + 3O2 + 8OH- = 2NO-3+6NO- 2+ 4H2O
������������� ��������Ϣ��֪X��N��Y��O��Z��Na��Q��Al��M��Cl��
��1��NH3�ĵ���ʽ��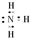
��2��A��ͬ���ڴ�������ԭ�Ӱ뾶�ݼ�������Rb>I����ȷ��B��RbM��ֻ�������Ӽ�������C����̬�⻯�����ȶ�����ǽ�����ǿ���йأ�M>I ����ȷ��D��Rb��Q��M������������Ӧ��ˮ����ֱ�Ϊǿ������������ǿ�ᣬ���Կ�������������Ӧ����ȷ��
��3����������ɵâ�ʽN2��g��+2Al��s��="2" AlN��s�� ��H=+2akJ/mol�� ��ʽ3C��s��+ Al 2O3��s��+ N2��g��=" 2" AlN��s��+3CO��g����H=+2bkJ/mol,�ɢ�ʽ-��ʽ�ɵ�Ŀ�귽��ʽ��3C��s��+ Al 2O3��s��= 2Al��s��+3CO��g�� ��H=" -(2a-2b)" kJ/mol��
��4��X��Y��ɵ�һ����ɫ����ΪNO������������8:3�ı�����Ӧ��8NO + 3O2 + 8OH- = 2NO-3+6NO- 2+ 4H2O
���㣺������Ԫ���ƶ�Ϊ���忼���˹����Ӧ�á�����ʽ��Ԫ�������ɡ�����ʽ��д�����֪ʶ����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ���ɻ�ͭ��(��Ҫ�ɷ�CuFeS2)ұ��ͭ����Ҫ�������£�
��1������A�еĴ�����Ⱦ���ѡ�������Լ��е�_______���ա�
a��ŨH2SO4 b��ϡHNO3 c��NaOH��Һ d����ˮ
��2����ϡH2SO4��������B��ȡ����������Һ���μ�KSCN��Һ��ʺ�ɫ��˵����Һ�д��� (�����ӷ���)��������Һ�л�����Fe2���ķ����� (ע���Լ�������)��
��3������ͭұ����ͭ�Ļ�ѧ��Ӧ����ʽΪ ��
��4����CuSO4��ҺΪ�������Һ���д�ͭ(��Al��Zn��Ag��Pt��Au������)�ĵ�⾫��������˵����ȷ���� ��
a������ȫ��ת��Ϊ��ѧ��
b����ͭ�ӵ�Դ����������������Ӧ
c����Һ��Cu2���������ƶ�
d������������ɻ���Ag��Pt��Au�Ƚ���
��5�����÷�Ӧ2Cu+O2+2H2SO4=2CuSO4+2H2O���Ʊ�CuSO4�������÷�Ӧ���Ϊԭ��أ��������缫��ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��Ϊ��ѧ��ѧ�г��������ʣ�����֮���ת����ϵ����ͼ�����ֲ�������ȥ����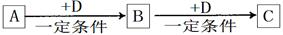
�Իش�
��1����D�Ǿ��������Եĵ��ʣ������ڶ����ڵ��������Ԫ��AΪ ����Ԫ�ط��ţ���
��2����D�ǽ������ʣ�D�ڳ�ʪ�Ŀ���������������ʴ��C��Һ�ڱ���ʱӦ�����������D��ֹ����ʣ�������D��C��Һ�ڿ����б��ʵ����ӷ���ʽΪ ����D���Ȼ����ˮ��Һ���ɲ����ղ����� ��
��3����A��B��C��Ϊ��������Ҿ����ؿ��к�����ߵĽ���Ԫ��E������Һ��A��C��Ӧ����B����д��Bת��ΪC�����п��ܵ����ӷ���ʽ ��
��4�����ڣ�1�����Ƴ���A������ڣ�3����E���ʵĻ����11.9gͶ��һ������ˮ�г�ַ�Ӧ��A��E��û��ʣ�࣬���ռ�����״���µ�����vL����������Һ����μ���Ũ��Ϊ2mol?L-1��H2SO4��Һ����100mLʱ��ɫ�����ﵽ���������v= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����������һ��dz���Ҫ�Ļ�����������Ƶõ�һ�ֹ�������������200�������ʷ�ˣ����ڶ�Ĺ��������й�����������Ϊֹ��Ȼ�õ��㷺��Ӧ�á�
��1����H2O2������ͬ�ĵ���������˫ԭ�ӷ����� ��д���֣���
��2�����Ӻ�ˮ����ȡ�⣬�轫�⻯���ɵ��ʵ⡣д��������������H2O2����I�����ӵ����ӷ���ʽ ��
��3��Na2O2��K2O2��CaO2��BaO2����������������H2O2��Ŀǰʵ������ȡH2O2��ͨ������ij�ֹ���������������ϡH2SO4���ò����˺��ã��������ʺϵĹ��������� ���ѧʽ����
��4����H2O2���������ȼ�ϵ�����������֪0��4molҺ̬�£�N2H4����������Һ̬H2O2��Ӧ������N2��g����H2O��g�����ų�256��6kJ����������÷�Ӧ���Ȼ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����X��Y��Z����ͬ�ֶ�����Ԫ�أ���ת����ϵ����ͼ��ʾ����Ӧ����δ�������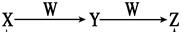
��1����X����ɫ��������ˮ�Ĵ̼�����ζ���壬Z�Ǻ���ɫ���壬��Y��W��Ӧ����Z�Ļ�ѧ����ʽ��_______________________________________________________��
��2����X�����ֶ�����Ԫ�أ���������Ԫ�ص�ԭ�ӵ�������֮�͵�����һ��Ԫ��ԭ�ӵ�������������W�dz�����������X��ϡ��Һת��ΪY�����ӷ���ʽ��
��
��3����X�ǿ�������Ҫ�ɷ�֮һ��Wԭ�ӵ��������������ڲ�������Ķ�������Y��W��Ӧ����0.1 mol Zʱ����Ӧ��ת�Ƶĵ�����Ϊ__________________________��
��4����Y�ǰ�ɫ��״������WΪ�ռ��0.1mol Y������W��Ӧ����Zʱ�����ռ������Ϊ �� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʳ�üӵ��ε������������£�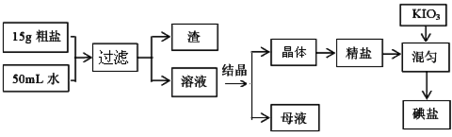
��1������ĸҺ���Ƿ���SO42�����ӵķ����� ��
��2������ʱ���õ��IJ��������У� �� �� ��
��3�����ұ�GB546061-92�涨���κ��������Ե�ƣ���������Ʒ��40mg/Kg������Ʒ��30mg/Kg��ij��ʵ��ʱ��һ�ɾ��������з���5g���Σ�����1mL0��001mol/L��KIO3��Һ������ȣ���100����º��1 h�����õ��β�Ʒ���õ��εĺ�����ԼΪ______mg/Kg������һλС�������Ƿ�Ϊ�ϸ��Ʒ ��ѡ��ϸ��ϸ���
��4����������Ƿ���е���أ����Լ��鷽���������Խ����мӻ�ԭ��KCNS���䷴Ӧ���£�6IO3��+5CNS��+H++2H2O��3I2+5HCN+5SO42��������ʱ��������KCNS�⣬����Ҫʹ�õ�һ���Լ��� ��
��5��ʹ�õ���ʱ��Ҫ��ֹ���£�����Ϊ���� �����еĵ������560�濪ʼ�ֽ������ɫ���壬ʣ������м������ữ����������Һ�л�ɫ�������ɣ��� ��������ˮ�У�Ҳ�ᷴӦ�������ֵ��ʣ�����һ������ɫ���壬�ڷ�Ӧ�����Һ�еμӷ�̪��Һ�Ժ�ɫ������������Ϣд���١����з�����Ӧ�Ļ�ѧ����ʽ��
�� ��
�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��Ԫ�����ڱ��н�����ǽ����ķֽ紦�������ҵ�
| A���Ͻ� | B���뵼����� | C������ | D��ũҩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���д�С�Ƚ���ȷ����
| A�����Ӱ뾶��Na+>Mg2+>Al3+>Cl����ԭ�Ӱ뾶��Na>Mg>Al>Cl |
| B���ȶ��ԣ�HF>H2O>NH3>CH4�� ��ԭ�ԣ� HF < H2O < NH3< CH4 |
| C�����ԣ�CsOH>KOH>Mg(OH)2>NaOH �� �����ԣ�Cs>K>Mg>Na |
| D�����ԣ�HClO>H2SO4>H2CO3���ǽ�����Cl>S>C |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com