
����Ŀ����Һ�еĻ�ѧ��Ӧ��������ӷ�Ӧ������Ҫ��ش��������⣺
��1���μ��(���϶�Na2CO3��NaCl)������ֲ���������������ӷ���ʽ��ʾ�μ�سʼ��Ե�ԭ��____________________________________��
��2����֪ˮ����ƽ��2H2O![]() H3O����OH������ˮ�м���NaHSO4���壬ˮ�ĵ���ƽ��________�ƶ���������Һ��________�ԡ�
H3O����OH������ˮ�м���NaHSO4���壬ˮ�ĵ���ƽ��________�ƶ���������Һ��________�ԡ�
��3����ȡpH���������ȵ�NaOH��Һ�Ͱ�ˮ�ֱ��ˮϡ��m����n����ϡ�ͺ�pH����ȣ���m________ n(���������������)��
��4�������£���pH��6��CH3COOH��CH3COONa�Ļ����Һ�У���ˮ���������c(OH��)��________mol��L��1��
��5����֪�������£�NH3��H2O�ĵ���ƽ�ⳣ��Kb��1.75��10��5��H2CO3�ĵ���ƽ�ⳣ��Ka1��Ka2�ֱ�Ϊ4.4��10��7��5.6��10��11, ��������Kb��__________(����Ka1�� ���� Ka2��)����֪NH4HCO3��Һ���������________�ԡ�
���𰸡� CO![]() ��H2O
��H2O![]() HCO
HCO![]() ��OH�� ���� �� �� 1��10��8 Ka1 ��
��OH�� ���� �� �� 1��10��8 Ka1 ��
����������1��̼����ˮ�⣬��Һ�Լ��ԣ�����μ�سʼ��Ե�ԭ����CO32����H2O![]() HCO3����OH������2����ˮ�м���NaHSO4���壬������Ũ������ˮ�ĵ���ƽ�������ƶ���������Һ����������3��ϡ�����дٽ�һˮ�ϰ��ĵ��룬�����������ʵ������ӣ�����Ҫʹϡ�͵ĺ��pH��ȣ���ˮϡ�͵ı�����m��n����4�������£���pH��6��CH3COOH��CH3COONa�Ļ����Һ�д���ĵ���̶ȴ��ڴ������ˮ��̶��������ˮ���������c(OH��)��
HCO3����OH������2����ˮ�м���NaHSO4���壬������Ũ������ˮ�ĵ���ƽ�������ƶ���������Һ����������3��ϡ�����дٽ�һˮ�ϰ��ĵ��룬�����������ʵ������ӣ�����Ҫʹϡ�͵ĺ��pH��ȣ���ˮϡ�͵ı�����m��n����4�������£���pH��6��CH3COOH��CH3COONa�Ļ����Һ�д���ĵ���̶ȴ��ڴ������ˮ��̶��������ˮ���������c(OH��)��![]() ����5������Kb��Ka1������̼�������ˮ��̶ȴ���笠���ˮ��̶ȣ���NH4HCO3��Һ�Լ��ԡ�
����5������Kb��Ka1������̼�������ˮ��̶ȴ���笠���ˮ��̶ȣ���NH4HCO3��Һ�Լ��ԡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪NO��O2ת��ΪNO2�ķ�Ӧ�������£�
��2NO(g)![]() 2N2O2(g)���죩 ��H1��0 ƽ�ⳣ��K1
2N2O2(g)���죩 ��H1��0 ƽ�ⳣ��K1
��N2O2(g) +O2(g)![]() 2NO2(g) ������ ��H2��0 ƽ�ⳣ��K2
2NO2(g) ������ ��H2��0 ƽ�ⳣ��K2
����˵����ȷ����
A. ��Ӧ�����е������仯����ͼa��ʾ
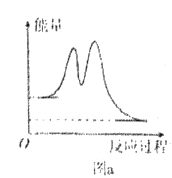
B. 2NO(g) +O2(g)![]() 2NO2(g)�ġ�H=-(��H1+��H2)
2NO2(g)�ġ�H=-(��H1+��H2)
C. 2NO(g)+O2(g)![]() 2NO2(g)��ƽ�ⳣ��K=K1/K2
2NO2(g)��ƽ�ⳣ��K=K1/K2
D. ��Ӧ�ڵ����ʴ�С����2NO(g)+O2(g)![]() 2NO2(g)�ķ�Ӧ����
2NO2(g)�ķ�Ӧ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��������У����÷�Һ©�����з�����ǣ�������
A.�ƾ���ˮ
B.���Ȼ�̼��ˮ
C.���ͺ�ֲ����
D.������Ȼ�̼
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��A��B��C��D��E���ֶ����ڵ�����Ԫ�أ����ǵ�ԭ���������ε�����A������Ԫ����ԭ�Ӱ뾶��С��Ԫ�أ�B��C��ɵ�ijһ�ֻ���������������ЧӦ�������£�����D����Ͷ��ˮ������ˮ���ҷ�Ӧ��������E�����dz��������壮�밴Ҫ��ش����м������⣺
��1��B��D��Ԫ�����Ʒֱ�Ϊ��_____��_____��
��2��E�����ڱ��е�λ��Ϊ��_____��
��3��C���ӵĽṹʾ��ͼΪ��_____��
��4��B��C��D����Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳��Ϊ_____����Ԫ�ط��ű�ʾ����
��5��B��E������������Ӧˮ��������Դ�ǿ������˳��Ϊ��_____���ö�Ӧ�Ļ�ѧʽ��ʾ��
��6��д��C��D����Ԫ����ɵ����������Ӹ�����Ϊ1:2��ֻ�����Ӽ��Ļ�����ĵ���ʽ��_____��
��7��д��E��ˮ��Ӧ�����ӷ���ʽ��_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��E��һ�ֻ���������,��ϳ�·�����£�
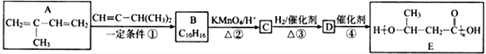
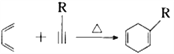
��֪����
��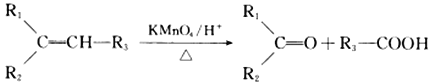 (R1��R2��R3��R����)
(R1��R2��R3��R����)
��ش��������⣺
��1��A�й����ŵ�������____________������ù����ŵ��Լ�Ϊ___________________��
��2����Ӧ�ٵĻ�ѧ����ʽ�ǣ�______________________________���䷴Ӧ����Ϊ________________���ڷ�Ӧ���У������Եõ���һ�ַ���ʽΪC10H16�Ļ������ṹ��ʽΪ___________________��
��3����֪��![]() ����Ϊ��ͪ�ᣬ��C��ϵͳ����������ӦΪ____________________��
����Ϊ��ͪ�ᣬ��C��ϵͳ����������ӦΪ____________________��
��4��д����Ӧ�ܵĻ�ѧ����ʽ��______________________________��
��5��C��ͬ���칹��X��������������
�ٳ����£���̼������Һ��Ӧ�ų����壻
���ܷ���������Ӧ�������������X����________�֡���˴Ź���������������ҷ����֮��Ϊ1��1��2��2����X�Ľṹ��ʽΪ________________��
��6������E�������ϳ�·�ߣ����һ����4һ��һ3һ��ͪ��Ϊ��ʼԭ���Ʊ�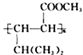 �ĺϳ�·��(���Լ���ѡ)_________________________________________��
�ĺϳ�·��(���Լ���ѡ)_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڷ�Ӧ�Ⱥ��Ȼ�ѧ��Ӧ����������ȷ����
A. 1 mol����ȼ��������̬ˮ�Ͷ�����̼ʱ���ų��������Ǽ����ȼ����
B. CO(g)��ȼ������283.0 kJ��mol-1����Ӧ2CO2(g)![]() 2CO(g)+O2(g) ��H=+2��283.0 kJ��mol-1
2CO(g)+O2(g) ��H=+2��283.0 kJ��mol-1
C. ������ȼ����Ϊ285.5 kJ��mol-1������ˮ���Ȼ�ѧ����ʽΪ2H2O(l)![]() 2H2(g)+O2(g) ��H=+285.5 kJ��mol-1
2H2(g)+O2(g) ��H=+285.5 kJ��mol-1
D. HCl��NaOH��Ӧ���к��Ȧ�H=-57.3 kJ��mol-1����H2SO4��Ca(OH)2��Ӧ���к��� ��H=2��(-57.3) kJ��mol-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)�ɹ�ҵ�ϳ���(���ΪH2��CO��������CO2)ֱ���Ʊ������ѣ��漰�����ĸ����淴Ӧ:
�״��ϳɷ�Ӧ:
(i)CO(g) +2H2(g)==CH3OH(g) ��H 1=-90.1kJ��mol-1��
(ii)CO2(g)+3H2(g)== CH3OH(g) +H2O(g) ��H2=-49.0kJ��mol-1��
ˮú���任��Ӧ:(iii)CO(g)+ H2O(g)==CO2(g)+ H2(g) ��H3=-41.1kJ��mol-1��
�����Ѻϳɷ�Ӧ:(iv)2CH3OH(g) ==CH3OCH3(g)+ H2O(g) ��H4=-24.5kJ��mol-1��
����H2��COֱ���Ʊ�������(��һ����Ϊˮ����)���Ȼ�ѧ����ʽΪ____________________________�����ݻ�ѧ��Ӧԭ������������ѹǿ��ֱ���Ʊ������ѷ�Ӧ��Ӱ��:___________________________________��
�ڷ�Ӧ( ii )��ƽ�ⳣ������ʽΪK=______________��
(2)���Զ�����ȼ�ϵ�أ����õ�ⷨ���������Ժ�����ˮ(��Ҫ����Cr2O72-)��ʵ����������ͼ2װ��ģ��÷�:
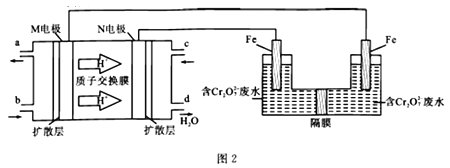
��M���(aΪCO2)�ĵ缫��ӦʽΪ________________________�����������ĵ缫��ӦʽΪ_______________________________________��
����д��������Cr2O72-ת��ΪCr3+�����ӷ�Ӧ����ʽ:__________________________��
����֪25 ��ʱ��Ksp[Cr(OH)3]=6.4��10-31��һ��������Ũ�ȡ�1��10-5 mol/L��Ϊ�����ӳ����ı���������ˮʱ�����Cr3+��Cr(OH)3��ʽ��ȥ������Һ��pH=6ʱ��c(Cr3+)=______��Cr3+ ___ (��ǡ���)��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��ʵ�飬���ܴﵽʵ��Ŀ�ĵ��ǣ���ѡ���жԱ���Һ��Ũ���������ͬ��( )
ʵ�鷽�� |
|
|
|
|
Ŀ�� | A����֤�����¶ȿɼӿ�H2O2�ֽ� | B����֤����Ӧ��Ũ�ȶ�ƽ���Ӱ�� | C���Ƚ�Cu2+��Fe3+��H2O2�ֽ����ʵ�Ӱ�� | D���Ƚ���������ǿ�� |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ܡ����Ƚ������仯�����ڿ�ѧ�о���ҵ������Ӧ��ʮ�ֹ㷺���ش���������:
(1)�����������Ļ�̬ԭ�Ӻ���δ�ɶԵ�����������________��
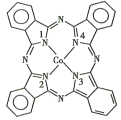
(2)̪ݼ�ܷ��ӵĽṹ��ʽ��ͼ��ʾ����������Ϊ�����ӣ�̪�ܷ�������������ͨ����λ����ϵĵ�ԭ�ӵı����_______(��1��2��3��4)�����ַǽ���ԭ�ӵĵ縺���ɴ�С��˳��Ϊ____(����Ӧ��Ԫ�ط��ű�ʾ)����ԭ�ӵ��ӻ��������Ϊ____��
(3)Fe(CO)x�������³�Һ̬���۵�Ϊ-20.5�����е�Ϊ103���������ڷǼ����ܼ����ݴ˿��ж�Fe(CO)x����������_____(�������)���������Fe(CO)x������ԭ�Ӽ۵������������ṩ������֮��Ϊ18����x=_______��
(4)NiO��FeO�ľ���ṹ�������Ȼ��Ƶ���ͬ��Ni2+��Fe2+�����Ӱ뾶�ֱ�Ϊ69pm��78pm�����۵�NiO____FeO(����>����<������=��)��ԭ����____________��
(5)NiAs�ľ����ṹ��ͼ��ʾ:
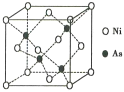
�������ӵ���λ��Ϊ__________��
���������ӵ�������ֵΪNA�������ܶ�Ϊpg��cm-3����þ����������Ni2+֮��ľ���Ϊ______cm��(д���������ʽ)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com