
| c(OH-) |
| c(H+) |
| Fe3+ | Al3+ | Fe2+ | Mg2+ | |
| ��ʼ����ʱ | 1.5 | 3.3 | 6.5 | 9.4 |
| ������ȫʱ | 3.7 | 5.2 | 9.7 | 12.4 |
| c(OH-) |
| C(H+) |
| c(OH-) |
| C(H+) |
| [CH3COO-]?[H+] |
| [CH3COOH] |
| [CH3COOH]?[OH-] |
| [CH3COO-] |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��pH��ֽ |
| B��NaOH |
| C��Na2CO3 |
| D����ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���������DZ��ʹ���Ļ���� |
| B��������Ǵ��������� |
| C������������ɫ��ζ��Һ�� |
| D�����������ܽ���ˮ���Ҵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����С | B������ |
| C������ | D����������Ҳ���ܼ�С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����0.1 mol?L-1BA��Һ�У�c��A-��+c��H+��=c��BOH��+c��B+�� |
| B������0.1 mol?L-1 BOH��Һϡ����0.001 mol?L-1����Һ��pH=9 |
| C������һ��������������Һ��Ϻ�pH=7������Һ�У�c��A-����c��B+�� |
| D��������������Һ�������1��1��ϣ�����Һ�У�c��A-����c��B+����c��H+����c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
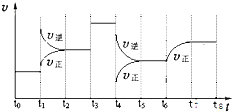 T��ʱ����ij�ݻ���Ϊ2L�ܱ������г���2molN2��4molH2���ڴ��������·�����Ӧ��N2��g��+3H2��g��?2NH3��g����H=-92.0kJ/mol��t0ʱ�̣�����ƽ��������NH3����Ϊ2mol����t1ʱ�̿�ʼ���ı䷴Ӧ��һ����������ϵ�з�Ӧ������ʱ��仯���������ͼ��ʾ��
T��ʱ����ij�ݻ���Ϊ2L�ܱ������г���2molN2��4molH2���ڴ��������·�����Ӧ��N2��g��+3H2��g��?2NH3��g����H=-92.0kJ/mol��t0ʱ�̣�����ƽ��������NH3����Ϊ2mol����t1ʱ�̿�ʼ���ı䷴Ӧ��һ����������ϵ�з�Ӧ������ʱ��仯���������ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������ѹǿ |
| B����������ƽ��Ħ������ |
| C�����������ܶ� |
| D�������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����֪2H2��g��+O2��g���T2H2O��g����H=-483.6 kJ/mol����1mol����ȼ�շų�����Ϊ483.6 kJ |
| B����C��ʯī��s���TC�����ʯ��s����H=+11.9 kJ?mol-1��֪��ʯī�Ƚ��ʯ�ȶ� |
| C��ͬ��ͬѹ�£�H2��g��+Cl2��g���T2HCl��g���ڹ��պ͵�ȼ�����ġ�H��ͬ |
| D����ѧ�仯��һ���������������ı仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com