
Ԫ�����ڱ�����ʽ���ֶ�������ͼ������Ԫ�����ڱ���һ���֣�������ѧ��ѧ������ʽԪ�����ڱ����ش��������⣺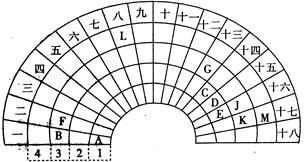
��1��Ԫ��C�����������ĵ���ʽΪ ��Ԫ��G�����ӽṹʾ��ͼΪ ��
��2��Ԫ��Lλ�����ڱ��ĵ� �壬 1mol/L LM2��Һ500ml��0.4 mol K����ǡ����ȫ��Ӧ�����ӷ���ʽΪ_______________________________________��
��3��������X����B��E��G����Ԫ����ɣ���ˮ��Һ��_______�ԣ�ԭ����___________
_____________________________________________________(�����ӷ���ʽ��ʾ)
��4��D��G��ɵĻ�����GD�������������������Ԫ������ҵ����G�������C���ʺ�D�����ڸ������Ʊ�GD������G���������C���ʵ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5����ȡ�ֹ�Ĺ����У�SiO�Ƿ�Ӧ�м�����������ʱSiO��NaOH��Һ��Ӧ������֮һ�ǹ����ƣ��Ļ�ѧ����ʽ______________________________________________
��1��
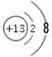
��2��VIII 10Fe2++6Br - +8Cl2=10Fe3++3Br2+16Cl-
��3�����ԣ���1�֣� AlO2-+2H2O 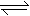 Al��OH��3+OH-
Al��OH��3+OH-
��4��Al2O3+3C+N2  2AlN+3CO
2AlN+3CO
��5�� SiO+2NaOH=Na2SiO3+H2��
�����������������Ԫ�����ڱ���Ԫ�ص����λ�ã���AΪ�⡢BΪ�ơ�CΪ̼��DΪ����EΪ����FΪþ��GΪ����JΪ��KΪ�ȡ�LΪ����MΪ�塣
��1��Ԫ��C�����������Ϊ������̼������ʽΪ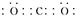 ��Ԫ��GΪ����Al3�������ӽṹʾ��ͼΪ
��Ԫ��GΪ����Al3�������ӽṹʾ��ͼΪ ��
��
��1��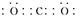
��2��Ԫ��LΪ����λ�����ڱ��ĵ�VIII�� 1mol��L��1 LM2(FeBr2)��Һ500mL��0.4 mol K����(Cl2)�����ʵ���֮��Ϊ5��4��ǡ����ȫ��Ӧ����Ϊ��ԭ�ԣ�Fe2��> Br�������Fe2���ȱ�������2Fe2��+ Cl2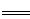 Fe3�� + 2Cl����
Fe3�� + 2Cl����
ÿĦ��Fe2����Ӧ�õ�0.5molCl2������4:2��Ӧ����ʣ��1.5mol��Cl2��Br����Ӧ��2Br��+ Cl2 Br2 + 2Cl����
Br2 + 2Cl����
ע��ֻ��3mol��Br����Cl2�����ˣ���ΪCl2���㣬�������ܷ�Ӧ��10Fe2�� +8Cl2+6Br�� 10Fe3�� +16Cl�� +3Br2��
10Fe3�� +16Cl�� +3Br2��
��3��������X��NaAlO2������ǿ�������Σ� ˮ���Լ��ԣ�AlO2��+2H2O Al(OH)3+OH����
Al(OH)3+OH����
��4��������GD��AlN��G����������Al2O3��C�����ǣ�D�����ǵ�����Al2O3��C���ʵ���֮��Ϊ1��3���ô���ϵ�������ȼ���Al2O3ϵ��Ϊ1��CΪ3����֪AlNΪ2��N2Ϊ1������ԭ���غ㣬��֪������3molCO�����������Al2O3+3C+N2  2AlN+3CO��
2AlN+3CO��
��5��SiO��NaOH��Һ��Ӧ����Ϊ����֮һ�����ƣ��軯�ϼ��ɣ�2��Ϊ��4�����ϼ������ߣ���Ȼ�л��ϼ۽��ͣ�������֪��ֻ����Ԫ�ػ��ϼۿ��Խ��ͣ��Ƴ���һ��������������ƽ��SiO+2NaOH=Na2SiO3+H2����
���㣺���鿼��Ԫ�����ڱ���Ӧ�ã�����Ԫ�ص�λ�ü����ʵĿ��飬���ս����ԵıȽϡ�ԭ�Ӱ뾶�ıȽϡ�������ԭ��Ӧ�ȼ��ɽ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
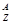 X��ʾԭ�ӣ�
X��ʾԭ�ӣ�
(1)����ԭ�ӵ�������N��______��
(2)AXn������x�����ӣ���������ӵ�������N��______��
(3)AXn������x�����ӣ���������ӵ�������N��______��
(4)12C16O2�����������N��________��
(5)A2��ԭ�Ӻ�����x�����ӣ���������Ϊm����n g A2���������ӵ����ʵ���Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��CΪ������Ԫ�أ������ڱ���������λ����ͼ��ʾ��A��C��Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������Bԭ�Ӻ�������������������ȡ�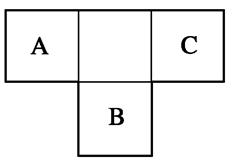
��1��A��B��C����Ԫ�ص����Ʒֱ�Ϊ__________��________��________��
��2��Bλ��Ԫ�����ڱ��е�________���ڵ�________�塣
��3��C��ԭ�ӽṹʾ��ͼΪ________��C�ĵ�����H2��Ӧ�Ļ�ѧ����ʽΪ_______________________________________________________________________��
��4��д��A����̬�⻯����B������������Ӧˮ���ﷴӦ�Ļ�ѧ����ʽ__________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���û�ʯȼ�Ͽ��ɡ��ӹ����̲�����H2S������ȡ�������ȼ����ֻ�����
��1����ҵ�Ͽ������ΪK2O��M2O3��2RO2��nH2O�������ϴ�����ȡ��������
����֪Ԫ��M��R��λ��Ԫ�����ڱ��е������ڣ�����Ԫ��ԭ�ӵ�������֮��Ϊ27����R��ԭ�ӽṹʾ��ͼΪ________��
�ڳ����£�������M���ʷ�����Ӧ����________������ţ���
a��CuSO4��Һ b��Fe2O3 c��Ũ���� d��NaOH��Һ e��Na2CO3����
��2������H2S������ȡ�����ķ����ж��֡�
�ٸ����ȷֽⷨ
��֪��H2S��g�� H2��g����
H2��g���� S2��g��
S2��g��
�ں����ܱ������У����Ʋ�ͬ�¶Ƚ���H2S�ֽ�ʵ�顣��H2S��ʼŨ�Ⱦ�Ϊc mol��L��1�ⶨH2S��ת���ʣ��������ͼ��ͼ��aΪH2S��ƽ��ת�������¶ȹ�ϵ���ߣ�b���߱�ʾ��ͬ�¶��·�Ӧ������ͬʱ����δ�ﵽ��ѧƽ��ʱH2S��ת���ʡ���ͼ����985��ʱH2S��������Ӧ�ֽ��ƽ�ⳣ��K��________��˵�����¶ȵ����ߣ�����b������a�ƽ���ԭ��____________________________________��
�ڵ绯ѧ��
�÷�������̵�ʾ��ͼ���¡���Ӧ���з�Ӧ��������������Һ������ʽ����Ŀ����______________________________��
��Ӧ���з�����Ӧ�Ļ�ѧ����ʽΪ____________________����Ӧ�����Һ������أ�����ܷ�Ӧ�����ӷ���ʽΪ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
X��Y��Z��Q��R�����ֶ�����Ԫ�أ�ԭ��������������X��Y��Ԫ����������������֮�;�Ϊ0��Q��Xͬ���壻Z��R�ֱ��ǵؿ��к�����ߵķǽ���Ԫ�غͽ���Ԫ�ء�
��ش��������⣺
��1������Ԫ��ԭ�Ӱ뾶�ɴ�С��˳����(дԪ�ط���)________��
��2��������ijЩԪ����ɵĻ�����A��B��C��D������ת����ϵ��
A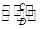 B(��ˮ��Һ�н���)
B(��ˮ��Һ�н���)
����C������ˮ�����Ե����壻D�ǵ���ɫ���塣
д��C�Ľṹʽ________��D�ĵ���ʽ________��
�����A��B��������Ԫ����ɣ�BΪ���Բ������A�Ļ�ѧʽΪ____________����Aת��ΪB�����ӷ���ʽΪ_____________________________________________��
�����A������Ԫ����ɣ�B������Ԫ����ɣ�A��B��Һ���Լ��ԡ������ӷ���ʽ��ʾA��Һ�Լ��Ե�ԭ��________________________________________��A��BŨ�Ⱦ�Ϊ0.1 mol��L��1�Ļ����Һ�У�����Ũ���ɴ�С��˳����__________________�������£��ڸ���Һ�еμ�ϡ����������ʱ�����ʵ���Ҫ�ɷ���_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
п��Zinc���ǵ��ġ��������Ľ�������������������ͭ�����ִ���ҵ�ж��ڵ�������в���ĥ��Ĺ��ס�
��.ʪ����п
ij��ұ�����̿�����ͼ���Ա�ʾ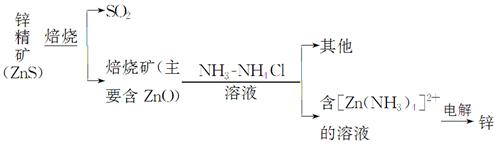
��1��ZnS���շ�Ӧ�Ļ�ѧ����ʽΪ_____________________________________��
��2������������пһ���ĵ缫��ӦʽΪ_______________________________��
��3������п��������һ�����������������������п���������Ʒ�Ӧ�����ӷ���ʽΪ_________________________________________________________��
����֪����п�ᡱ�Ļ�ѧʽ��д��H2[Zn��OH��4]��
��.��
��п�������ĵ���������洦�ɼ�����п�̸ɵ�ء�п�̼��Ե�ء�п�յ�صȡ�
��4��п�̼��Ե�أ��Զ�������Ϊ������п��Ϊ����������������ҺΪ���Һ������������ŵ��������ص㣬����õ��㷺Ӧ�á�����ܷ�ӦʽΪZn��2MnO2��2H2O=2MnO��OH����Zn��OH��2��
���Ե���У�����п��Ƭ״�ı����״���ŵ���_______________________��������ӦʽΪ________________________________________________________________________��
��5������п�յ�أ���ͼ����﮵����ȣ�п��������صĴ�������������������ɱ���﮵�ص�һ�룬������ȫû�й��ȱ�ը�İ�ȫ�������õ�ص��ܷ�ӦΪ2Zn��O2=2ZnO���������ҺΪKOH��Һ�����ĵ缫��ӦʽΪ__________________________�����Ըõ��Ϊ��Դ���ö��Ե缫�����������Һ��Ϊ��֤������10.8 g��������������Ҫ________L����������ɱ�״��������õ�ء�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Q��W��X��Y��ZΪ����ԭ�����������Ķ�����Ԫ�ء�
��֪����Qԭ�Ӻ�����������ڵ��Ӳ���������W��ɵĻ���������������Ҫ�ɷ֣�
��W��Y��X��Y��ɵĻ������ǻ������ų��Ĵ�����Ⱦ�
��Y��Z���γɵ�������Ϊ30��38���������ӻ����
��1��W�����ڱ���λ�ã�________��������Ϊ38��Y��Z�γɻ�����ĵ���ʽ��________��
��2����ҵ�ϳ�XQ3����H��0�����д�ʩ���ܼӿ췴Ӧ���ʣ�����ʹԭ��ת����һ������ߵ���________��
| A�������¶� |
| B����������� |
| C����XQ3��ʱ�����ȥ |
| D������Ӧ��ϵѹǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E��F��Ϊ������Ԫ�أ���ԭ��������������A��ԭ�Ӱ뾶��С��Ԫ�أ�B������������ˮ����������⻯�ﷴӦ�γ����ӻ�����ף�A��D������ԭ�Ӹ�����4��1�γɻ������ң����ҷ����к���18�����ӣ�E��Bͬ���壬C����������F�����������һ�����Ӳ㣬�ҿ��γ����Ӹ�����Ϊ2��1�����ӻ��������
(1)D��ԭ�ӽṹʾ��ͼΪ ________ �����ĵ���ʽΪ___________ ��E�����ڱ��е�λ��Ϊ ��
(2)����˵����ȷ���� ��
�ٻ������ҷ�����ֻ���м��Թ��ۼ�
��C��D��E��Fԭ�Ӱ뾶�ɴ�С��˳��ΪC>D>E>F
��B��E�γɵ��⻯���У�B���⻯����ȶ�
�ܻ�����ͻ���������������Ӽ����ۼ�
(3)��Fȼ�յIJ���ͨ��BaCl2��HNO3�Ļ����Һ�У����ɰ�ɫ�������ų���ɫ���壬����һ�����ӷ���ʽ��ʾ�÷�Ӧ __________________ ��
(4)д��һ��������Ԫ�ع��ɵ�10��������18��������Ӧ�����ӷ���ʽ ____________________________ ��
(5)����Һ�� (����ԡ��������ԡ������ԡ�)��ԭ���� (�����ӷ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��X��Y��ZԪ�ص�ԭ������������������Ϣ������⣺
| Ԫ��A | ���ܼ��ϵĵ�������� |
| Ԫ��C | ij�ֺ���ԭ�ӵ�������Ϊ18��������Ϊ10 |
| Ԫ��X | ���������õİ뵼����� |
| Ԫ��Y | �䵥��Ϊ����ɫ���壬���������������ˮ���¶ȼ� |
| Ԫ��Z | 3d�ܼ�����4��δ�ɶԵ��� |
 H+(aq)+CH3COO��(aq) ��H��+akJ/mol
H+(aq)+CH3COO��(aq) ��H��+akJ/mol�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com