
| A£® | ĻņijČÜŅŗÖŠ¼ÓČė³ĪĒåŹÆ»ŅĖ®£¬ČÜŅŗ±ä»ė×Ē£¬ŌņøĆČÜŅŗŅ»¶Øŗ¬ÓŠCO32- | |
| B£® | ĻņijČÜŅŗÖŠ¼ÓČėAgNO3ČÜŅŗ£¬Éś³É°×É«³Įµķ£¬øĆČÜŅŗÖŠŅ»¶Øŗ¬ÓŠCl- | |
| C£® | ĻņijČÜŅŗÖŠ¼ÓČėŃĪĖįĖį»ÆµÄBaCl2ČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É£¬øĆČÜŅŗÖŠŅ»¶Øŗ¬ÓŠSO42- | |
| D£® | ĻņijČÜŅŗÖŠ¼ÓČė2µĪKSCNČÜŅŗ£¬ČÜŅŗ²»ĻŌŗģÉ«£¬ŌŁĻņČÜŅŗÖŠ¼øµĪŠĀÖʵÄĀČĖ®£¬ČÜŅŗ±äĪŖŗģÉ«£¬øĆČÜŅŗÖŠŅ»¶Øŗ¬ÓŠFe2+ |
·ÖĪö A£®ČÜŅŗÖŠæÉÄÜŗ¬ÓŠĢ¼ĖįĒāøłĄė×Ó”¢ŃĒĮņĖįøłĄė×Ó»ņŃĒĮņĖįĒāøłĄė×Ó£¬²»Ņ»¶Øŗ¬ÓŠĢ¼ĖįøłĄė×Ó£»
B£®øĆ°×É«³ĮµķæÉÄÜĪŖĢ¼ĖįŅų”¢ŃĒĮņĖįŅų£¬²»Ņ»¶Øŗ¬ÓŠĀČĄė×Ó£»
C£®Éś³ÉµÄ°×É«³ĮµķæÉÄÜĪŖĀČ»ÆŅų£¬ŌČÜŅŗÖŠ²»Ņ»¶Øŗ¬ÓŠĮņĖįøłĄė×Ó£»
D£®¼ÓČėĮņĒč»Æ¼ŲČÜŅŗŗó²»±äÉ«£¬µĪČėŃõ»ÆŠŌµÄĀČĖ®ŗó±äĪŖŗģÉ«£¬“Ó¶ųæÉÖ¤Ć÷ŌČÜŅŗÖŠŗ¬ÓŠŃĒĢśĄė×Ó£®
½ā“š ½ā£ŗA£®ĻņijČÜŅŗÖŠ¼ÓČė³ĪĒåŹÆ»ŅĖ®£¬ČÜŅŗ±ä»ė×Ē£¬æÉÄÜÉś³ÉĮĖĢ¼ĖįøĘ»ņŃĒĮņĖįøĘ³Įµķ£¬ŌČÜŅŗæÉÄÜŗ¬ÓŠSO32-”¢HCO3-£¬²»Ņ»¶Øŗ¬ÓŠCO32-£¬¹ŹA“ķĪó£»
B£®ĻņijČÜŅŗÖŠ¼ÓČėAgNO3ČÜŅŗ£¬Éś³É°×É«³Įµķ£¬øĆ°×É«³ĮµķæÉÄÜĪŖĢ¼ĖįŅų”¢ŃĒĮņĖįŅų£¬ŌČÜŅŗÖŠæÉÄÜŗ¬ÓŠCO32-”¢SO32-£¬²»Ņ»¶Øŗ¬ÓŠCl-£¬¹ŹB“ķĪó£»
C£®ĻņijČÜŅŗÖŠ¼ÓČėŃĪĖįĖį»ÆµÄBaCl2ČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É£¬øĆ°×É«³ĮµķæÉÄÜĪŖĮņĖį±µ»ņĀČ»ÆŅų£¬ŌņŌČÜŅŗÖŠæÉÄÜŗ¬ÓŠAg+£¬²»Ņ»¶Øŗ¬ÓŠSO42-£¬¹ŹC“ķĪó£»
D£®ĻȵĪČėĮņĒč»Æ¼ŲČÜŅŗŗóČÜŅŗ²»ĻŌŹ¾ŗģÉ«£¬ĖµĆ÷ČÜŅŗÖŠ²»ŗ¬ĢśĄė×Ó£¬µĪČėĀČĖ®ŗóČÜŅŗ±äĪŖŗģÉ«£¬ĖµĆ÷ŌČÜŅŗÖŠŗ¬ÓŠŃĒĢśĄė×Ó£¬¹ŹDÕżČ·£»
¹ŹŃ”D£®
µćĘĄ ±¾Ģā漲鳣¼ūĄė×ӵļģŃé·½·Ø£¬ĢāÄæÄѶČÖŠµČ£¬ŹģĻ¤³£¼ūĄė×ӵĊŌÖŹ¼°¼ģŃé·½·ØŹĒ½ā“š±¾ĢāµÄ¹Ų¼ü£¬×¢Ņā¼ģŃéĄė×ÓŹ±Ó¦ÅųżĘäĖüĄė×ÓµÄøÉČÅ£¬Č·±£¼ģŃé·½°øµÄŃĻĆÜŠŌ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÕżĪģĶéŗĶÕż¼ŗĶé | B£® | ±½ŗĶŅŅĶé | ||
| C£® | ¶Ō¶ž¼×±½ŗĶ2£¬2-¶ž¼×»ł±ūĶé | D£® | ¼×±½ŗĶ2-¼×»ł¶”Ķé |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
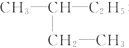 ŗĶ
ŗĶ
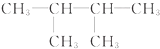 ŗĶ
ŗĶ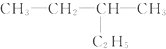
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CH3COOD£¬C2H5OD | B£® | CH3COONa£¬C2H5OD£¬HOD | ||
| C£® | CH3COONa£¬C2H5OH£¬HOD | D£® | CH3COONa£¬C2H5OD£¬H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼×Ķé | B£® | ŅŅĻ© | C£® | ŅŅĶé | D£® | ±ūĻ© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CuS | B£® | FeCl2 | C£® | FeS | D£® | SO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ·ŠµćøßµĶ£ŗHI£¾HBr£¾HCl£¾HF | |
| B£® | ČČĪČ¶ØŠŌ“󊔣ŗHF£¾H2O£¾NH3£¾PH3 | |
| C£® | ČŪµćøßµĶ£ŗ½šøÕŹÆ£¾Ź³ŃĪ£¾½šŹōÄĘ£¾±ł | |
| D£® | Ī¢Į£°ė¾¶“󊔣ŗS2-£¾Cl-£¾F-£¾Na+£¾Al3+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓŠ»śĪļ²»Ņ»¶Ø¶¼²»ČÜÓŚĖ® | |
| B£® | ÓŠ»śĪļ¶¼ŹĒ¹²¼Ū»ÆŗĻĪļ | |
| C£® | ÓŠ»śĪļ¶¼ŹĒ“ÓÓŠ»śĢåÖŠ·ÖĄė³öĄ“µÄĪļÖŹ | |
| D£® | ÓŠ»śĪļ²»¾ß±øĪŽ»śĪļµÄŠŌÖŹ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com