
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | O |
| 1 | �� | |||||||
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | ||||
| 4 | �� | |||||||
| 5 | �� |
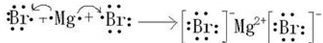 ��
������ Ԫ�������ڱ��е�λ�ã���֪����H������Na������Mg������Al������C������N������O������Cl������Br������I��
��1��N������Ϊ����Br�����Ϊ+7�ۣ�
��2���ٺ͢�����Ԫ�ص�ԭ�Ӱ�1��1��ɵij���������Ϊ�������⣻
��3�����Ӳ�Խ�࣬���Ӱ뾶Խ������ͬ�����Ų������ӣ�ԭ������������Ӱ뾶С��
��4���ڡ��ܵ�����������ˮ���ﷴӦ����ƫ�����ƺ�ˮ��
��5���ۺ͢���ɵĻ�����Ϊ�Ȼ�þ��
��6���������ữ�£�����˫��ˮ��������Ϊ���ʣ���֪�����ӱ�������
��� �⣺Ԫ�������ڱ��е�λ�ã���֪����H������Na������Mg������Al������C������N������O������Cl������Br������I��
��1��N������Ϊ����Br�����Ϊ+7�ۣ���ۺ�����Ļ�ѧʽΪ���ʴ�Ϊ������HBrO4��
��2���ٺ͢�����Ԫ�ص�ԭ�Ӱ�1��1��ɵij���������Ϊ�������⣬�ṹʽΪH-O-O-H���ʴ�Ϊ��H-O-O-H��
��3�����Ӳ�Խ�࣬���Ӱ뾶Խ������ͬ�����Ų������ӣ�ԭ������������Ӱ뾶С�������Ӱ뾶�ɴ�С��˳��ΪCl-��O2-��Mg2+��Al3+��
�ʴ�Ϊ��Cl-��O2-��Mg2+��Al3+��
��4���ڡ��ܵ�����������ˮ���ﷴӦ����ƫ�����ƺ�ˮ�����ӷ�ӦΪAl��OH��3+OH-=AlO2-+2H2O���ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��5���ۺ͢���ɵĻ�����Ϊ�Ȼ�þ���õ���ʽ��ʾ��������γɹ���Ϊ ��
��
�ʴ�Ϊ�� ��
��
��6���������ữ�£�����˫��ˮ��������Ϊ���ʣ���֪�����ӱ����������ӷ�ӦΪ2H++H2O2+2I-=2H2O+I2���ʴ�Ϊ��2H++H2O2+2I-=2H2O+I2��
���� ���⿼��λ�á��ṹ�����ʵĹ�ϵ��Ϊ��Ƶ���㣬����Ԫ�ص�λ�á����ʡ�Ԫ��������Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬��Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
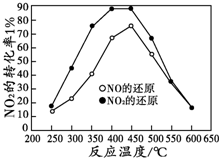 ���������е�CO��NOX�Ѿ���Ϊ��������Ҫ��Ⱦ�ʹ��ϡ���ȴ����ܽ�CO��NOx��̼�⻯����ת���������ʣ��Ӷ���������β����Ⱦ��
���������е�CO��NOX�Ѿ���Ϊ��������Ҫ��Ⱦ�ʹ��ϡ���ȴ����ܽ�CO��NOx��̼�⻯����ת���������ʣ��Ӷ���������β����Ⱦ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �۰��� ������
�۰��� ������ ��${\;}_{17}^{37}$Cl ��${\;}_{17}^{35}$Cl �����
��${\;}_{17}^{37}$Cl ��${\;}_{17}^{35}$Cl ����� ������ԭ�Ӻ�10���ӵ����������ӷ���H3O+������ԭ�Ӻ�18���ӵĹ��ۻ����ﻯѧʽH2O2��
������ԭ�Ӻ�10���ӵ����������ӷ���H3O+������ԭ�Ӻ�18���ӵĹ��ۻ����ﻯѧʽH2O2���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 14C���������������ļ�����12C��14C��Ϊͬλ�� | |
| B�� | �����������Ѽ�·�ˡ����������γɵ����ܽ��ж����ЧӦ | |
| C�� | ��Ԫ�����ڱ��Ľ����ͷǽ����ֽ��߸���Ѱ�Ұ뵼����� | |
| D�� | ������һ�գ���ˮ�����գ���ȡ֭���������϶������ص���ȡ���ڻ�ѧ�仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����100mL2mol•L-1���� | B�� | ����̼���ƹ��� | ||
| C�� | �μ�����CuCl2��Һ | D�� | �����¶� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 18gD2O��18gH2O�к��е���������Ϊ10NA | |
| B�� | 0.5mol/L����������Һ�к��е�SO32-�����ʵ���Ϊ0.5mol | |
| C�� | ����������ˮ��Ӧʱ������0.1mol����ת�Ƶĵ�����Ϊ0.2NA | |
| D�� | ����ͭƬ��100mL18mol/L��Ũ���ᷴӦ��ת�Ƶĵ�����Ϊ1.8NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | N2O�ṹʽ�ɱ�ʾΪN=N=O | |
| B�� | O3���ӳ�ֱ���� | |
| C�� | CH2=CH-CHO������̼ԭ�Ӿ�����sp2�ӻ� | |
| D�� | ��ͬѹǿ�£�HCOOH�е��CH3OCH3�ߣ�˵��ǰ���Ǽ��Է��ӣ������ǷǼ��Է��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ���ʣ��������ʣ� | ���� | |
| A | KNO3���壨NaCl�� | ��ˮ�ܽ⡢�����ᾧ�����ȹ��ˡ�ϴ�ӡ����� |
| B | NaCl���壨KNO3�� | ��ˮ�ܽ⡢����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����� |
| C | FeCl3��Һ��NH4Cl�� | �������ɡ����� |
| D | NH4Cl��Һ��FeCl3�� | �μӰ�ˮ�����ٲ�������Ϊֹ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com