
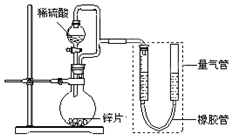
 ijͬѧ�������ͼ��ʾװ�ã����ּг�װ������ȥ������װ�ÿ����������ж���ʵ���о���
ijͬѧ�������ͼ��ʾװ�ã����ּг�װ������ȥ������װ�ÿ����������ж���ʵ���о���| ��� | V��H2SO4��/mL | c��H2SO4��/mol•L-1 | t/s |
| �� | 40 | 1 | t1 |
| �� | 40 | 4 | t2 |
���� ��1����п��ϡ���ᷴӦ��������п��������
��t1��t2��˵��ʵ���Ӧ���ʽϴ��Ũ�ȵ�Ӱ���жϣ�
�۲�õķ�Ӧ���ʾ���������ʵ���Ӧ�����ݣ���ѧ��Ӧ������������������п���γ�ԭ��ض��ӿ췴Ӧ���ʣ��ݴ��жϣ�
��2���ٽ��Zn+2H+=Zn2++H2��������ΪVL���㣻
�ڶ���ǰ������Һ��߶ȣ�
�۸�������װ���жϣ�
��3���ٷ���������ʴӦ�ڼ��Ի�������Һ�н��У�
�ڷ���������ʴʱ�������������������Ҷ�Һ���½������Һ��������
��� �⣺��1����п��ϡ���ᷴӦ��������п����������Ӧ�����ӷ���ʽΪZn+2H+=Zn2++H2����
�ʴ�Ϊ��Zn+2H+=Zn2++H2����
��t1��t2��˵��ʵ���Ӧ���ʽϴ���Ũ�Ȳ�ͬ��˵������������һ��ʱ����ѧ��Ӧ�����淴Ӧ��Ũ�ȵ����������
�ʴ�Ϊ������������һ��ʱ����ѧ��Ӧ�����淴Ӧ��Ũ�ȵ����������
���ɷ�Ӧ���ʴ�������ʵ���Ӧ�����ݿ�֪�������ʱ�����п�γ�ԭ��أ�ʹ��Ӧ���������������ʿ����DZ�п�����õģ�ʯī������ͭ�ȣ�
�ʴ�Ϊ��abc��
��2���ٲ���������ΪVL����״�����������ʵ���Ϊ$\frac{V}{22.4}$mol������Zn+2H+=Zn2++H2����֪��Zn������Ϊ$\frac{V}{22.4}$mol��65g/mol������п��mg�����п���Ĵ���Ϊ$\frac{65V}{22.4m}$��100%��
�ʴ�Ϊ��$\frac{65V}{22.4m}$��100%��
�ڶ������ܶ���ʱ��Ҫע������������ܵ�Һ��߶���ƽ����������Һ����ƽ��
�ʴ�Ϊ�������������ܵ�Һ��߶���ƽ����������Һ����ƽ��
����Ŀװ���У�װ������ķ�Һ©����ͨ������������ƿ�ͷ�Һ©���Ϸ���������ѹǿ��ͬ����ʵ��������ƿ�е����ϡ���������Բⶨ���������Ӱ�죬
�ʴ�Ϊ����Ӱ�죻
��3���ٸ��������Ի������Ի���������������ʴ��
�ʴ�Ϊ��c��
��������ʴ�������������������Ҷ�Һ���½������Һ��������
�ʴ�Ϊ���������Ҷ�Һ���½������Һ��������
���� ���⿼�黯ѧ��Ӧ�����Լ������ĵ绯ѧ��ʴ��ʵ��̽��������ʱע��ʵ�����ݵĴ���������������ʵ��ԭ��������ʵ������ó�ʵ����ۣ�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�����и߶��ϵ�һ���¿���ѧ���������棩 ���ͣ������
���з�Ӧ��mA��g����nB��g�� pC��g�����ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C������������С����
pC��g�����ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C������������С����
��1���÷�Ӧ���淴ӦΪ________��Ӧ������ȡ����ȡ�������m��n________p���>������������<������
��2����ѹʹ�����������ʱ��A����������__________�������������С�����䡱����ͬ��
��3��������B��ά��������䣩����A��ת����____________��
��4���������¶ȣ���ƽ��ʱB��C��Ũ��֮�Ƚ�____________��
��5�������������ƽ��ʱ��������������ʵ���__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�������и߶��ϵ�һ���¿���ѧ���������棩 ���ͣ������
��1�� �����£�ijˮ��ҺM�д��ڵ������У�Na+��A2-��HA-��H+��OH-�����ڵķ�����H2O��H2A����������ش��������⣺
��д����H2A�ĵ��뷽��ʽ__________________��
������ҺM��10mL 2 mol��L-1NaHA��Һ��2mol��L-1NaOH��Һ�������϶��ã�����ҺM��pH ________7 ���>������<����=��������ҺM�и�����Ũ�ȹ�ϵ��ȷ����__________��
A��c��Na+����c��A2-����c��OH-����c��H+��
B��c��HA-��+c��H2A��+c��H+��=c��OH-��
C��c��A2-��+c��HA-��+c��H2A��=1mol��L-1
D��c��A2-��+c��HA-��+c��OH-��=c��Na+��+c��H+��
��2�� CO2���������壬����NaOH��Һ���յõ�Na2CO3��NaHCO3
��Na2CO3�׳ƴ����CO32-ˮ���ʹ��ˮ��Һ�ʼ��ԣ���д��CO32-ˮ������ӷ���ʽ�����Եڶ���ˮ�⣩______________����ˮ�ⷴӦ��ƽ�ⳣ������ˮ�ⳣ�����ı���ʽΪKh=__________��
����֪25��ʱ��Kh=2��10-4mol/L������Һ��c��HCO3-����c��CO32-��=2��1ʱ��������Һ��pH=__________��
��0.1mol/L Na2CO3��Һ��c��OH-����c��H+��=__________���ú�c��HCO3-����c��H2CO3���Ĺ�ϵʽ��ʾ��
���� Na2CO3��Һ�м���������������������壬��д����ص����ӷ���ʽ__________________��
��3��ʵ���ҿ���NaOH��Һ����NO2����ӦΪ2NO2+2NaOH===NaNO3+NaNO2+H2O����0.2 mol NaOH ��ˮ��Һ�� 0.2molNO2ǡ����ȫ��Ӧ��1L��ҺA����ҺBΪ0.1mol��L-1��CH3COONa��Һ��������Һ��c��NO3-����c��NO2-����c��CH3COO-���ɴ�С��˳��Ϊ__________������֪HNO2�ĵ��볣��Ka=7.1��10-4mol��L-1��CH3COOH�ĵ��볣��Ka=1.7��10-5mol��L-1������ʹ��ҺA����ҺB��pH��ȵķ�����____________��
a������ҺA�м�����ˮ b������ҺA�м�����NaOH
c������ҺB�м�����ˮ d������ҺB�м�����NaOH
��4��ֱ���ŷź�SO2���������γ����꣬Σ�������������Ƽ�ѭ�������ѳ������е�SO2������Һ����SO2�Ĺ����У�pH��n��SO32-����n��HSO3-��
n��SO32-����n��HSO3-�� | 91:9 | 1:1 | 9:91 |
pH | 8.2 | 7.2 | 6.2 |
�����ϱ��ж�NaHSO3��Һ��____�ԣ��û�ѧƽ��ԭ�����ͣ�______________________��
�ڵ�����Һ������ʱ����Һ�г�����Ũ�ȹ�ϵ��ȷ���ǣ�ѡ����ĸ��_________________��
A��c��Na+��=2c��SO32-��+c��HSO3-��
B��c��Na+����c��HSO3-����c��SO32-����c��H+��=c��OH-��
C��c��Na+��+c��H+��=c��SO32-��+c��HSO3-��+c��OH-��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�������и߶��ϵ�һ���¿���ѧ���������棩 ���ͣ�ѡ����
�����£����и���������ָ��������һ���ܴ����������
A����������Һ�У�Na+��K+��Cl-��HCO3-
B����ɫ��Һ�У�Al3+��NH4+��Cl-��HCO3-
C��pH=1����Һ�У�ClO-��SO42-��Fe2+��K+
D����ˮ�����c��OH-��=10��14mol�� L-1����Һ�У�CH3COO-��SO42-��Na+��NH4+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����Ӧ�Ļ��С��100kJ/mol | |
| B�� | �淴Ӧ���һ��С��100kJ/mol | |
| C�� | ����Ӧ���С��100kJ/mol | |
| D�� | ����Ӧ��ܱ��淴Ӧ��ܴ�100kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1 mol H2��0.5 mol O2��Ӧ�ų�����������H2��ȼ���� | |
| B�� | ���������������������ֱ���ȫȼ�գ���ͬ�����£�ǰ�߷ų��������� | |
| C�� | ˮ�еĸ�բ�����ӵ�Դ�ĸ�����������ӵ��������������� | |
| D�� | ������Ũ�������ۻ����ɱ����ڲ�������ʴ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
| ʵ�� ��� | �¶� ���棩 | ���� ������g�� | ���Ը��������Һ | |
| �����mL�� | Ũ�ȣ�mol/L�� | |||
| 1 | 25 | 0.5 | 4.00 | 0.1000 |
| 2 | 50 | 0.5 | 4.00 | 0.1000 |
| 3 | 25 | 0.5 | 4.00 | 0.0100 |
| 4 | 25 | 0 | 4.00 | 0.1000 |
| ʵ�� ��� | ��Һ��ɫ����ʱ�䣨min�� | ||
| �� 1 �� | �� 2 �� | �� 3 �� | |
| 1 | 14.0 | 13.0 | 11.0 |
| 2 | 6.5 | 6.7 | 6.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ���� | ���� | ���͡����� |
| A | ����¶���ڳ�ʪ�����е�Fe���м���������ϡ�����ַ�Ӧ������KSCN��Һ | ��Һ�ʺ�ɫ | ϡ���ὫFe����ΪFe3+ |
| B | ��Na2CO3��Һ��ͨ������CO2 | ��Һ����� | ������Na2CO3���� |
| C | Al������Ũ������ | ������ | Al��Ũ�����жۻ����γ����ܵ�����Ĥ |
| D | �ò�����պȡŨ��ˮ�㵽��ɫʯ����ֽ�� | ��ֽ���ɫ | Ũ��ˮ�ʼ��� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com