
��15�֣���ͼ��ʾijЩ��������֮���ת����ϵ����Ӧ��������ȥ��������A��DΪ��̬�ǽ������� ��B��CΪ�������ʣ�BΪ��ɫ��E��F��G��HΪ�����GΪ��ɫҺ�壬�ס��ҡ�����������Ϊ��Һ����Ϊǿ���ϡ��Һ���١��ھ�Ϊ��ҵұ��B�ķ�Ӧ��
��ش��������⣺
��1��F��ͬ������Ԫ����ɣ�������ʳ�����ף�Ҳ�Ǵ�����Ⱦ��֮һ����Ⱦ��Դ��Ҫ�� �����ڴ����п��γɱ�����Ӧ�Ļ�ѧ����ʽΪ ����Ӧ��Ҳ�������ɱ�����Ӧ�����ӷ���ʽΪ ��
��2���ɷ�Ӧ���ƵõĽ���B�����ʣ���ҵ���� ������B�ᴿ��99.95%���ϡ������Ȼ�ԭ�ƵõĽ���C��Ҳ�����ʣ��ڳ�ʪ�����Ի����У�C��������ʴ����ѧ����ʽΪ ��C����Ӧ�۵Ĵ������������ɺ�ɫ����H�ɼ���ʴ��
��3��������Һ���н������ӵķ����� ;
��4��E���ɵ���ֱ�ӻ��ϵõ�����E�Ļ�ѧʽΪ ����Ӧ�ٵĻ�ѧ����ʽΪ ��
��1����ʯȼ�ϵ�ȼ�գ����������֣���1�֣�
2SO2+O2+2H2O=2H2SO4���ֿ��ɵ÷֣���2�֣�
SO2+H2O2=2H++SO42-��2�֣�
��2����⾫����2�֣���2Fe+O2+2H2O=2Fe��OH��2��1�֣���
4Fe��OH��2+O2+2H2O=4Fe��OH��3��1�֣���
2Fe��OH��3=Fe2O3��xH2O+��3-x��H2O��1�֣���Ҳ�ɽ���������ʽȫ���ּӺͣ�
��3��ȡ��������Һ���Թ��У�����KSCN��Һ�Ժ�ɫ֤����Fe3+��1�֣�����ȡһ��������Һ���뵽ʢ������KMnO4��Һ���Թ��У���Һ��ɫ��֤����Fe2+��1�֣�
��4��Cu2S��1�֣���Cu2S+O22Cu+SO2��2�֣�
����:


 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д� ������ϵ�д�
������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����



| ||
| ||
| 5 |
| 2 |
| 5 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�������ʡ��У����һģ�����ۣ���ѧ���� ���ͣ������
��15�֣���ͼ��ʾijЩ��������֮���ת����ϵ����Ӧ��������ȥ��������A��DΪ��̬�ǽ������ʣ�B��CΪ�������ʣ�BΪ��ɫ��E��F��G��HΪ�����GΪ��ɫҺ�壬�ס��ҡ�����������Ϊ��Һ����Ϊǿ���ϡ��Һ���١��ھ�Ϊ��ҵұ��B�ķ�Ӧ��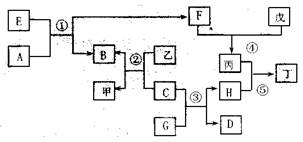
��ش��������⣺
��1��F��ͬ������Ԫ����ɣ�������ʳ�����ף�Ҳ�Ǵ�����Ⱦ��֮һ����Ⱦ��Դ��Ҫ�� �����ڴ����п��γɱ�����Ӧ�Ļ�ѧ����ʽΪ ����Ӧ��Ҳ�������ɱ�����Ӧ�����ӷ���ʽΪ ��
��2���ɷ�Ӧ���ƵõĽ���B�����ʣ���ҵ���� ������B�ᴿ��99.95%���ϡ������Ȼ�ԭ�ƵõĽ���C��Ҳ�����ʣ��ڳ�ʪ�����Ի����У�C��������ʴ����ѧ����ʽΪ ��C����Ӧ�۵Ĵ������������ɺ�ɫ����H�ɼ���ʴ��
��3��������Һ���н������ӵķ����� ;
��4��E���ɵ���ֱ�ӻ��ϵõ�����E�Ļ�ѧʽΪ ����Ӧ�ٵĻ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�������ʡ��У����һģ�����ۣ���ѧ���� ���ͣ������
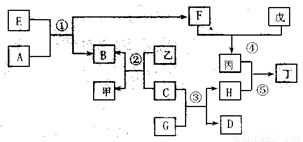
��15�֣���ͼ��ʾijЩ��������֮���ת����ϵ����Ӧ��������ȥ��������A��DΪ��̬�ǽ������� ��B��CΪ�������ʣ�BΪ��ɫ��E��F��G��HΪ�����GΪ��ɫҺ�壬�ס��ҡ�����������Ϊ��Һ����Ϊǿ���ϡ��Һ���١��ھ�Ϊ��ҵұ��B�ķ�Ӧ��
��ش��������⣺
��1��F��ͬ������Ԫ����ɣ�������ʳ�����ף�Ҳ�Ǵ�����Ⱦ��֮һ����Ⱦ��Դ��Ҫ�� �����ڴ����п��γɱ�����Ӧ�Ļ�ѧ����ʽΪ ����Ӧ��Ҳ�������ɱ�����Ӧ�����ӷ���ʽΪ ��
��2���ɷ�Ӧ���ƵõĽ���B�����ʣ���ҵ���� ������B�ᴿ��99.95%���ϡ������Ȼ�ԭ�ƵõĽ���C��Ҳ�����ʣ��ڳ�ʪ�����Ի����У�C��������ʴ����ѧ����ʽΪ ��C����Ӧ�۵Ĵ������������ɺ�ɫ����H�ɼ���ʴ��
��3��������Һ���н������ӵķ����� ;
��4��E���ɵ���ֱ�ӻ��ϵõ�����E�Ļ�ѧʽΪ ����Ӧ�ٵĻ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
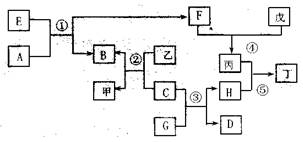
��ͼ��ʾijЩ��������֮���ת����ϵ����Ӧ��������ȥ��������A��DΪ��̬�ǽ������� ��B��CΪ�������ʣ�BΪ��ɫ��E��F��G��HΪ�����GΪ��ɫҺ�壬�ס��ҡ�����������Ϊ��Һ����Ϊǿ���ϡ��Һ���١��ھ�Ϊ��ҵұ��B�ķ�Ӧ��
��ش��������⣺
��1��F��ͬ������Ԫ����ɣ�������ʳ�����ף�Ҳ�Ǵ�����Ⱦ��֮һ����Ⱦ��Դ��Ҫ�� �����ڴ����п��γɱ�����Ӧ�Ļ�ѧ����ʽΪ ����Ӧ��Ҳ�������ɱ�����Ӧ�����ӷ���ʽΪ ��
��2���ɷ�Ӧ���ƵõĽ���B�����ʣ���ҵ���� ������B�ᴿ��99.95%���ϡ������Ȼ�ԭ�ƵõĽ���C��Ҳ�����ʣ��ڳ�ʪ�����Ի����У�C��������ʴ����ѧ����ʽΪ ��C����Ӧ�۵Ĵ������������ɺ�ɫ����H�ɼ���ʴ��
��3��������Һ���н������ӵķ����� ;
��4��E���ɵ���ֱ�ӻ��ϵõ�����E�Ļ�ѧʽΪ ����Ӧ�ٵĻ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com