���㣺̽�����ʵ���ɻ�������ʵĺ���,�Ƶ���Ҫ������
ר�⣺ʵ��̽�������ݴ�����
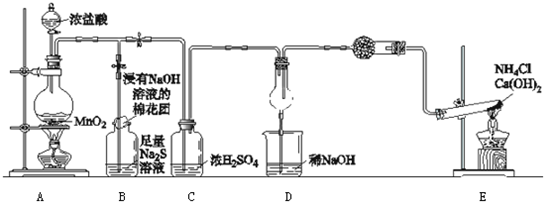
��������װ��ͼ��֪��ʵ��ԭ��Ϊ��������м������ᣬת��Ϊ������������̼����ȥ��Ӧ���ɵĶ�����������Eװ�õ�����ȷ�����ɵĶ�����̼�����������ݶ�����̼����������������̼���Ƶ������������������ֵ�����������
��1��������Ҫ�ⶨ������̼������������װ��Ӧ���������ã���ʵ��֮ǰӦ����װ�õ������ԣ�
��2��ʵ��ⶨ������̼����������������̼���Ƶ�������Ҫ����������������Ҫ֪����Ʒ������������Ӧ��Ҫ��������ƽ������Ʒ������������Eװ�õ�����ȷ�����ɵĶ�����̼����������Fװ��Ŀ���Ƿ�ֹ�����е�ˮ������������̼����Eװ���У�Ӱ����������
��3��ʵ��ⶨNa
2CO
3��Na
2SO
3������и���ֵĺ�������Ʒ���ᷴӦ���ɵ�����Ϊ������̼�Ͷ����������壬����Bװ�ó�ȥ������������Eװ�õ�����ȷ�����ɵĶ�����̼�����������ݶ�����̼����������������̼���Ƶ�������Ϊ���������������Բⶨ����ĸ��ţ�װ��C��������Ʒ����Һ��֤���������Ƿ�����
��4��ʵ��ԭ���dz�ȥ��Ӧ���ɵĶ�����������Eװ�õ�����ȷ�����ɵĶ�����̼�����������ݶ�����̼����������������̼���Ƶ����������Խ���װ��E�еĶ�����̼Ҫ���������������װ��B�������dz�ȥ�����еĶ��������Լ�ȫ�����ն������������ն�����̼�Ҳ������ɶ�����̼��װ��C����������֤���������Ƿ������װ��D�������Ǹ������壻
��5��װ���ڻ�������ֶ�����̼��Ӧʹ������̼ȫ����װ��E��ҩƷ���գ���ͨ������Ŀ���Ŀ�����ž�װ���ڵĶ�����̼��ʹ���ɵĶ�����̼�ܹ�ȫ������װ��E��ҩƷ���գ����ڿ����к��ж�����̼��Ӧ�ȳ�ȥ�����еĶ�����̼��������ȥ�����еĶ�����̼�����²ⶨ������̼����������������̼���Ƶ�������������ⶨ��Na
2S0
3��������
��6��װ��E��ʵ�����ʱ����4.4gΪ������̼�����������ʵ���Ϊ
=0.1mol������̼Ԫ���غ��֪�������̼���Ƶ�����Ϊ0.1mol��106g/mol=10.6g�������������Ƶ�����Ϊ23.2g-10.6g=12.6g�������������ʵ���Ϊ
=0.1mol���ݴ˼��㣮
���
�⣺������֪ʵ��ԭ��Ϊ��������м������ᣬת��Ϊ������������̼����ȥ��Ӧ���ɵĶ�����������Eװ�õ�����ȷ�����ɵĶ�����̼�����������ݶ�����̼����������������̼���Ƶ������������������ֵ�����������
��1��������Ҫ�ⶨ������̼������������װ��Ӧ���������ã���ʵ��֮ǰӦ����װ�õ������ԣ�
�ʴ�Ϊ������װ�õ������ԣ�
��2��ʵ��ⶨ������̼����������������̼���Ƶ�������Ҫ����������������Ҫ֪����Ʒ������������Ӧ��Ҫ��������ƽ������Ʒ��������
����Eװ�õ�����ȷ�����ɵĶ�����̼����������Fװ��Ŀ���Ƿ�ֹ�����е�ˮ������������̼����Eװ���У�Ӱ����������
�ʴ�Ϊ��������ƽ����ֹ�����е�ˮ������������̼����Eװ���У�
��3����Ʒ���ᷴӦ���ɵ�����Ϊ������̼�Ͷ����������壬����Bװ�ó�ȥ������������Eװ�õ�����ȷ�����ɵĶ�����̼�����������ݶ�����̼����������������̼���Ƶ�����������װ��C����������Ʒ����Һ��֤���������Ƿ�B������ȫ��
�ʴ�Ϊ������SO
2�Ƿ�B������ȫ��
��4��ʵ��ԭ���dz�ȥ��Ӧ���ɵĶ�����������Eװ�õ�����ȷ�����ɵĶ�����̼�����������ݶ�����̼����������������̼���Ƶ��������ʽ���װ��E�еĶ�����̼Ҫ�������������������װ��B�������dz�ȥ�����еĶ��������Լ������ն������������ն�����̼�Ҳ������ɶ�����̼������ѡ�����Լ���֪Ӧѡ��c�����Ը��������Һ��װ��C����������֤���������Ƿ������װ��D�������Ǹ������壬ѡ���Լ�Ϊa��Ũ���ᣩ��E��Fװ������ʢ��ʯ�ҵĸ���ܣ�����Eװ�õ�����ȷ�����ɵĶ�����̼��������FΪ���ų������еĶ�����̼��ˮ��������װ��E������
�ʴ�Ϊ��c��a��g��
��5��װ���ڻ�������ֶ�����̼��Ӧʹ������̼ȫ����װ��E��ҩƷ���գ�����ͨ������Ŀ���Ŀ�����ž�װ���ڵĶ�����̼��ʹ���ɵĶ�����̼�ܹ�ȫ����װ��E��ҩƷ���գ����ڿ����к��ж�����̼��Ӧ�ȳ�ȥ�����еĶ�����̼��������ȥ�����еĶ�����̼�����²ⶨ������̼����������������̼���Ƶ�������������ⶨ��Na
2S0
3������������Na
2SO
3����ƫ�ͣ�����ʹ������ͨ��e����������Һ����ȥ���еĶ�����̼��
�ʴ�Ϊ��ʹ���ɵĶ�����̼�ܹ�ȫ��װ��E��ҩƷ���գ�e��ƫ�ͣ�
��6��װ��E��ʵ�����ʱ����4.4gΪ������̼�����������ʵ���Ϊ
=0.1mol������̼Ԫ���غ��֪�������̼���Ƶ�����Ϊ0.1mol��106g/mol=10.6g�������������Ƶ�����Ϊ23.2g-10.6g=12.6g�����ʵ���Ϊ
=0.1mol��������Na
2CO
3��Na
2SO
3�����ʵ���֮��Ϊ0.1mol��0.1mol=1��1��
�ʴ�Ϊ��1��1��
���������⿼��ѧ����ʵ��ԭ�������⡢������ɵIJⶨ�ȣ��Ѷ��еȣ�����ԭ���ǹؼ�����Ҫѧ��������ʵ�Ļ���֪ʶ���ۺ�����֪ʶ������������������




 ��������ϵ�д�
��������ϵ�д�

 �������������������۾��IJ��ϣ���д�������йط�Ӧ�Ļ�ѧ����ʽ��
�������������������۾��IJ��ϣ���д�������йط�Ӧ�Ļ�ѧ����ʽ��