

| �������� | �����¶ȣ��棩 | ����+����������g�� |
| �� | ���� | 62.2 |
| �� | T1 | 56.8 |
| �� | T2 | 49.6 |
| �� | T3 | 44.2 |
| �� | T4 | 44.2 |
| ||
| ||
| 106 |
| 10.6g |
| 18n |
| 18g |


 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��K+��Na+��Cl-��CO32- |
| B��Cu2+��Cl-��Na+��SO42- |
| C��Ca2+��Na+��Cl-��NO3- |
| D��Fe3+��NH4+��SCN-��HCO3-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
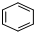 ��ȡ
��ȡ ��
��
 ����Ӧ�Ļ�ѧ����ʽΪ��
����Ӧ�Ļ�ѧ����ʽΪ���鿴�𰸺ͽ���>>
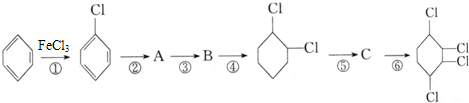
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
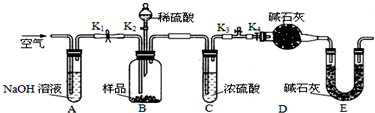
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����Ȼ�Ļ�ѧ����Ǿ������ϩ |
| B��������ë���ںϳ���ά |
| C��ͨ����������ʹ�ṹ�����ͱ�����ͽṹ |
| D�����������ڸ��ϲ��� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com