
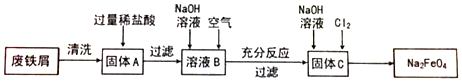
·ÖĪö ÓĆ·ĻĢśŠ¼£Øŗ¬ÉŁĮæĶ£©ÖĘČ”ŠĀŠĶ¾»Ė®¼ĮNa2FeO4µÄĮ÷³Ģ£ŗĒåĻ“·ĻĢśŠ¼±ķ±ķĆęµÄÓĶĪŪ£¬¼ÓČė¹żĮæµÄĻ”ŃĪĖį£¬ĢśČÜÓŚĻ”ŃĪĖį£¬Ķ²»ČÜ£¬¹żĀĖµĆµ½ĀČ»ÆŃĒĢśŗĶHClµÄ»ģŗĻČÜŅŗB£¬ĻņĘäÖŠ¼ÓČėĒāŃõ»ÆÄĘČÜŅŗ£¬ÖŠŗĶŃĪĖį£¬²¢Éś³ÉĒāŃõ»ÆŃĒĢś£¬ĶØČėæÕĘų£¬±»Ńõ»ÆĪŖĒāŃõ»ÆĢś£¬¹żĀĖµĆµ½µÄ¹ĢĢåCĪŖĒāŃõ»ÆĢś£¬¼ÓČėĒāŃõ»ÆÄĘŗĶĀČĘųµĆµ½²śĘ·Na2FeO4£¬¾Ż“Ė·ÖĪö£®
½ā“š ½ā£ŗ£Ø1£©¹ĢĢåAÖŠ¼ÓČėĻ”ŃĪĖįŗó·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ£ŗFe+2H+=Fe2++H2”ü£»ŃĒĢśĄė×ÓŅ×±»Ńõ»Æ£¬ČōŠč³¤Ź±¼ä±£“ę£¬æɼÓČė»¹Ō¼ĮĢś·Ū£»
¹Ź“š°øĪŖ£ŗFe+2H+=Fe2++H2”ü£»ĻņČÜŅŗÖŠ¼ÓČėÉŁĮæĢś·Ū£»
£Ø2£©ČÜŅŗBĪŖĀČ»ÆŃĒĢśŗĶ¶ąÓąµÄŃĪĖį£¬ŃōĄė×ÓĪŖ£ŗFe2+”¢H+£»ĻņBČÜŅŗÖŠ¼ÓČėĒāŃõ»ÆÄĘ£¬ĻČÉś³É°×É«³ĮµķĒāŃõ»ÆŃĒĢś£¬ĒāŃõ»ÆŃĒĢś±»Ńõ»Æ£¬·¢Éś·“Ó¦4Fe£ØOH£©2+O2+2H2OØT4Fe£ØOH£©3£¬Éś³ÉŗģŗÖÉ«µÄĒāŃõ»ÆĢś³Įµķ£¬¹ŹĻÖĻóĪŖ£ŗĻČ²śÉś°×É«³Įµķ£¬°×É«³ĮµķŃøĖŁ±äĪŖ»ŅĀĢÉ«£¬×īŗó±äĪŖŗģŗÖÉ«£»4Fe£ØOH£©2+O2+2H2OØT4Fe£ØOH£©3£»
¹Ź“š°øĪŖ£ŗFe2+”¢H+£»ĻČ²śÉś°×É«³Įµķ£¬°×É«³ĮµķŃøĖŁ±äĪŖ»ŅĀĢÉ«£¬×īŗó±äĪŖŗģŗÖÉ«£»4Fe£ØOH£©2+O2+2H2OØT4Fe£ØOH£©3£»
£Ø3£©ĻņĒāŃõ»ÆĢśÖŠ¼ÓČėNaOHČÜŅŗ²¢ĶØČėCl2æÉÖĘČ”Na2FeO4£¬·“Ó¦ĪŖ£ŗ2 Fe£ØOH£©3+10NaOH+3Cl2=2Na2FeO4+6NaCl+8H2O£»
¹Ź“š°øĪŖ£ŗ2 Fe£ØOH£©3+10NaOH+3Cl2=2Na2FeO4+6NaCl+8H2O£»
£Ø4£©Na2FeO4ÖŠÄĘŌŖĖŲ+1¼Ū£¬ŃõŌŖĖŲ-2¼Ū£¬»ÆŗĻ¼Ū“śŹżŗĶĪŖ0£¬ŌņFeµÄ»ÆŗĻ¼ŪŹĒ+6¼Ū£¬ĢśŌŖĖŲ+6¼Ū£¬Ņ׵Ƶē×Ó»ÆŗĻ¼Ū½µµĶ£¬¾ßÓŠŃõ»ÆŠŌ£¬æÉɱ¾śĻū¶¾£»
¹Ź“š°øĪŖ£ŗ+6£»Ńõ»Æ£»É±¾śĻū¶¾£®
µćĘĄ ±¾Ģāæ¼²éĮĖĪļÖŹµÄÖʱø£¬Ī§ČĘĢśÕ¹æŖ£¬Éę¼°Ńõ»Æ»¹Ō·“Ó¦·½³ĢŹ½µÄŹéŠ“£¬Į÷³ĢµÄ·ÖĪöµČ£¬ÕĘĪÕĪļÖŹŠŌÖŹŹĒ¹Ų¼ü£¬ĢāÄæÄѶČÖŠµČ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® |  ĪÅĘųĢåµÄĘųĪ¶ | B£® | 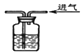 ÓĆÅØĮņĖįøÉŌļCO2 | ||
| C£® | 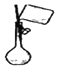 ĻņČŻĮæĘæÖŠ×ŖŅĘŅŗĢå | D£® | 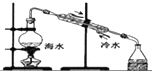 ÓĆŗ£Ė®ĢįČ”µĖ® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ł¢Ś¢Ū¢Ż | B£® | ¢Ł¢Ś¢Ü¢Ž | C£® | ¢Ś¢Ū¢Ü¢Ż | D£® | ¢Ū¢Ü¢Ż¢Ž |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
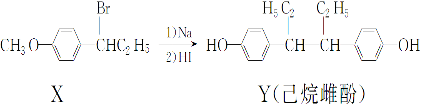
| A£® | ŌŚNaOH Ė®ČÜŅŗÖŠ¼ÓČČ£¬»ÆŗĻĪļX æÉ·¢ÉśĻūČ„·“Ó¦ | |
| B£® | ŌŚŅ»¶ØĢõ¼žĻĀ£¬»ÆŗĻĪļYæÉÓėÅØäåĖ®·¢ÉśČ”“ś·“Ó¦ | |
| C£® | ÓĆFeCl3ČÜŅŗ²»Äܼų±š»ÆŗĻĪļXŗĶY | |
| D£® | »ÆŗĻĪļYÖŠ²»ŗ¬ÓŠŹÖŠŌĢ¼Ō×Ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | c£ØSO42-£©=c£ØHS-£©=c£ØK+£©£¾c£ØOH-£©=c£ØH+£© | |
| B£® | c£ØNa+£©£¾c£ØK+£©£¾c£ØS2-£©£¾c£ØH+£©£¾c£ØOH-£© | |
| C£® | c£ØNa+£©=c£ØS2-£©+c£ØHS-£©+c£ØH2S£©+c£ØSO42-£© | |
| D£® | c£ØK+£©+c£ØNa+£©+c£ØH+£©=c£ØSO42-£©+c£ØS2-£©+c£ØHS-£©+c£ØOH-£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | A”¢BŌŖĖŲŠĪ³ÉµÄŅ»ĻµĮŠ»ÆŗĻĪļÖŠ£¬ĘäÖŠAŌŖĖŲÖŹĮæ·ÖŹżµÄ×ī“óÖµĪŖ25% | |
| B£® | ĖÄÖÖŌŖĖŲÖŠµēøŗŠŌ×ī“óµÄŹĒB | |
| C£® | CĖłŠĪ³ÉµÄĘųĢ¬Ēā»ÆĪļ£¬ŌŚĘäĶ¬Ö÷×åŌŖĖŲµÄĘųĢ¬Ēā»ÆĪļÖŠ·Šµć×īµĶ | |
| D£® | ĖÄÖÖŌŖĖŲÖŠµŚŅ»µēĄėÄÜ×īŠ”µÄŹĒD |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
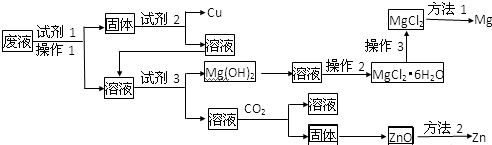
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ×¼Č·ĮæČ”20.00mLøßĆĢĖį¼ŲČÜŅŗ£¬æÉŃ”ÓĆ25mL¼īŹ½µĪ¶Ø¹Ü | |
| B£® | ½«Ė®¼ÓČČ£¬KwŌö“ó£¬pH²»±ä | |
| C£® | ÓƶčŠŌµē¼«µē½ā1LÅØ¶Č¾łĪŖ2mol/LµÄAgNO3ÓėCu£ØNO3£©2µÄ»ģŗĻČÜŅŗ£¬µ±ÓŠ0.2 mol µē×Ó×ŖŅĘŹ±£¬Ņõ¼«Īö³ö6.4g½šŹō | |
| D£® | NaAlO2µÄĖ®ČÜŅŗ¾¼ÓČČÅØĖõ”¢ÕōøÉ×ĘÉÕŗóÄܵƵ½NaAlO2¹ĢĢå |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¢Ū¢Ü¢Ż | B£® | ¢Ś¢Ū¢Ü | C£® | ¢Ł¢Ū¢Ü¢Ž | D£® | ¢Ł¢Ś¢Ū¢Ü |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com