
ʳ���к���һ������þ���������ʣ��ӵ����е����ʧ��Ҫ���������ʡ�ˮ�֡������е������Լ����ա����ȶ�����ġ���֪�������ԣ�
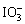 ��Fe3����I2����ԭ�ԣ�
��Fe3����I2����ԭ�ԣ� ��I����
��I����
3I2��6OH��
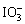 ��5I����3H2O��
��5I����3H2O��
KI��I2 KI3
KI3
��1��ijѧϰС��Լӵ��ν�������ʵ�飺ȡһ����ij�ӵ���(���ܺ���KIO3��KI��Mg2����Fe3��)������������ˮ�ܽ⣬����ϡ�����ữ����������Һ��Ϊ3�ݡ���һ����Һ�еμ�KSCN��Һ���Ժ�ɫ���ڶ�����Һ�м�����KI���壬��Һ�Ե���ɫ����CCl4��ȡ���²���Һ���Ϻ�ɫ����������Һ�м�������KIO3����μӵ����Լ�����Һ����ɫ��
�ټ�KSCN��Һ�Ժ�ɫ���ú�ɫ������_________���û�ѧʽ��ʾ����CCl4�����Ϻ�ɫ��������___________________���õ���ʽ��ʾ����
�ڵڶ�����Һ�м�������KI�����Ӧ�����ӷ���ʽΪ___________________________��______________________________________��
��2��KI��Ϊ�ӵ����ʳ���ڱ�������У����ڿ��������������ã�������������ʧ��
д����ʪ������KI��������Ӧ�Ļ�ѧ����ʽ��_____________________________��
��I2����KI��Һ���ڵ��������£����Ƶ�KI3��H2O����������Ϊʳ�μӵ���Ƿ���ʣ�______����ǡ�������˵������________________________________________��
��3��Ϊ����ӵ��Σ�����KI�����ȶ��ԣ��ɼ��ȶ������ٵ����ʧ�������������п�����Ϊ�ȶ�������___________________��
A��Na2S2O3 B��AlCl3 C ��Na2CO3 D��NaNO2
��4���Ժ�Fe2���϶��ʳ��(���費��Fe3��)����ѡ��KI��Ϊ�ӵ���������ʵ�鷽��������üӵ����е�Fe2����__________________________________________________________________________��
����������1����Fe3����SCN������ϲ����ж��֣��� ��
��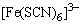 �ȣ�I2��CCl4��Һ���Ϻ�ɫ����Ӧ����Ϣ���������ԣ�
�ȣ�I2��CCl4��Һ���Ϻ�ɫ����Ӧ����Ϣ���������ԣ�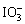 ��Fe3����I2����˵��
��Fe3����I2����˵��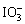 ��Fe3����������I������I2��
��Fe3����������I������I2��
��2��KI����ʪ��������������д��I����O2��H������Ҫ��ϵ����������ʴ������I2��KOH�ƺ�������(�ᷴӦ)��Ӧ���ǻ�����Ӧ��������I2��������KOH�������CO2��Ӧ��
KI3��H2O���ӵ�����⣬�Ƚ��ѷ�������ΪKI3��İ���������У������������¿��Ƶá��������в�����һʹ��ʵ����ȥȷ�����ٸ�����Ϣ����KI��I2 KI3�������䲻�ȶ��ԡ�
KI3�������䲻�ȶ��ԡ�
��3��������Ϣ����ԭ�ԣ� ��I�������ж�A��C�Ƚ��ѷ�����Ӧ����ʳ�γ�����Ҫ��Mg2����Fe3�����𣬼�Na2CO3��ʹ֮ת��Ϊ�����D��NaNO2������I�D��
��I�������ж�A��C�Ƚ��ѷ�����Ӧ����ʳ�γ�����Ҫ��Mg2����Fe3�����𣬼�Na2CO3��ʹ֮ת��Ϊ�����D��NaNO2������I�D��
��4��ʵ�鷽�����Ҫע��淶�ԣ�����ȡ�����롭�����ۡ�������ʵ��I�D��Fe2���ļ����и��ţ��ù�����ˮ�ֿ�������SCN������Ȼʵ�ʲ������жϣ������Գ̶Ⱥõ�ͬѧ��˵������³ʿ��������ȷ����ǿ��
���𰸡���1����Fe(SCN)3 
��IO3����5I����6H��=3I2��3H2O 2Fe3����2I��=2Fe2����I2
��2��O2��4I����2H2O=2I2��4KOH
�� KI3�����ȣ���ʪ�������²���I2��KI��KI������������I2��������
��3��AC
��4��ȡ�����üӵ�����������ˮ�У��������ữ���μ��������������磺��ˮ����������ȣ����ٵμ�KSCN���ڣ�����Ѫ��ɫ����üӵ����д���Fe2+��


 ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ�������������Ƿ��ѱ���������ѡ�Լ�(����������)����ȷ����(����)
A���Ȼ�������Һ(���軯����Һ)
B���⻯����Һ(������Һ)
C����ȩ(��ɫʯ����Һ)
D������������Һ(�Ȼ�����Һ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
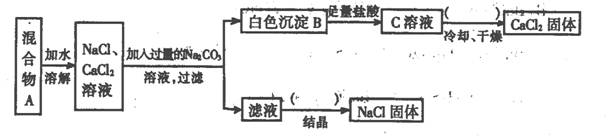 ijѧ�����������ʵ�飺
ijѧ�����������ʵ�飺
�ش��������⣺
(1)��ͬѧ��ʵ��Ŀ���� ��
(2)��ͼ�����ڵIJ��������Ϊ ��
(3)����ʵ�鷽���õ���NaCl�����п϶����� (�ѧʽ)���ʣ�Ϊ�˽����������������˵õ�����Һ�м��������� ��
��4��д������B�����ӷ���ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ס��������о���ѧϰС��Ϊ�ⶨ�������е�����ԭ�Ӹ����ȣ��������ʵ�����̣�


ʵ���У������Ƶĵİ����ž�ϴ��ƿǰ����װ���еĿ�����������ϴ��ƿ�������ռ�װ�ã�������������ͭ����Ӧ��Ϻ�ɫ������ͭת��Ϊ��ɫ��ͭ����ͼA��B��CΪ�ס�����С����ȡ����ʱ�����õ���װ�ã�DΪʢ��Ũ�����ϴ��ƿ��


��С���ã���Ӧǰ����ͭ������m1g������ͭ��Ӧ��ʣ����������m2g�����ɰ����ڱ�״���µ����V1L��
��С���ã�ϴ��ǰװ��D������m3g��ϴ����װ��D������m4g�����ɰ����ڱ�״���µ����V2L��
��ش��������⣺
��1�������a������ ��
��2�����Aװ�������ԵIJ����� ��������
��3���ס�����С��ѡ���˲�ͬ�ķ�����ȡ�������뽫ʵ��װ�õ���ĸ��ź��Ʊ�ԭ����д���±��Ŀո��С�
| ʵ��װ�� | ʵ��ҩƷ | �Ʊ�ԭ�� | |
| ��С�� | A | �������ơ����ᡢ����� | ��Ӧ�Ļ�ѧ����ʽΪ�١� ���������������������� �� |
| ��С�� | �� | Ũ��ˮ���������� | �û�ѧƽ��ԭ�������������Ƶ����ã� ���������� �� |
��4����С������������ݼ�����������е������ԭ�Ӹ���֮��Ϊ ��
��5����С������������ݼ�����������е������ԭ�Ӹ���������С������ֵ����ԭ���� ��Ϊ�ˣ���С����ԭ��ʵ��Ļ�����������һ��װ��ijҩƷ��ʵ������������ʵ�顣����ʵ��ǰ���ҩƷ�������仯�����ɰ�����������ó��˺�����ʵ��������ҩƷ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��̼����������ָͨ��һ���ķ�������ҵ�����в�����CO2������������á��������NaOH��Һ��������CO2���������������ͼ��ʾ����������������δ�������

�����йظ÷�������������ȷ����
A.�ܺĴ��Ǹ÷�����һ��ȱ��
B.���������У�ֻ��һ�����ʿ���ѭ������
C.����Ӧ���롱�����У��������ʵĻ��������������ᾧ������
D.�÷����ɼ���̼�ŷţ�������CO2���������Ʊ��״��Ȳ�Ʒ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ���������������������Ӧ����0.25g�����ĵ�314mL��������״�������������PCl3��PCl5�����ʵ���֮�Ƚӽ���
A��1��2 B��2��3 C��3��1 D��5��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij����С����MnO2��Ũ�����Ʊ�Cl2ʱ�����ø����չ�����SO2��NaOH��Һ����β���������մ�����
��1�������SO2�����NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��SO2+2NaOH = ________________��
��2����ӦCl2+Na2SO3+2 NaOH===2NaCl+Na2SO4+H2O�еĻ�ԭ��Ϊ________________��
��3������β��һ��ʱ�������Һ��ǿ���ԣ��п϶�����Cl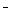 ��OH
��OH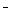 ��SO
��SO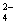 �������ʵ�飬̽��������Һ�п��ܴ��ڵ����������ӣ�������������CO2��Ӱ�죩��
�������ʵ�飬̽��������Һ�п��ܴ��ڵ����������ӣ�������������CO2��Ӱ�죩��
�� ����������� ��
����1��ֻ����SO32-������2���Ȳ�����SO32-Ҳ������ClO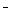 ������3��_____________��
������3��_____________��
�� ���ʵ�鷽��������ʵ�顣���ڴ����д��ʵ�鲽���Լ�Ԥ������ͽ��ۡ���ѡʵ���Լ���3moL L-1H2SO4��1moL
L-1H2SO4��1moL L-1NaOH��0.01mol
L-1NaOH��0.01mol L-1KMnO4������-KI��Һ����ɫʯ����Һ��
L-1KMnO4������-KI��Һ����ɫʯ����Һ��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ��������Һ���Թ��У��μ�3 moL | |
| ����2�� | |
| ����3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��22.4 Lij��̬����������������������ͭ����ȫ��Ӧ�����������Ϊ11.2 L(���������ͬ�����²ⶨ)����õ���������Ļ�ѧʽΪ( )
A.NO2 B.N2O3 C.N2O D.N2O4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͨ��״���£�X��Y��Z��������̬���ʡ�X�����Ԫ���ǵ�������ԭ�Ӱ뾶��С��Ԫ�أ�ϡ������Ԫ�س��⣩��Y��Z����Ԫ��R��ɣ���ӦY+2I-+2H+ I2+Z+H2O����ΪY�ļ�����Ӧ��
I2+Z+H2O����ΪY�ļ�����Ӧ��
(1)Y��Z�Ĺ�ϵ�ǣ�ѡ����ĸ��_______��
a.ͬλ�� b.ͬϵ�� c.ͬ�������� d.ͬ���칹��
(2)��Y�Ͷ�������ֱ�ͨ��Ʒ����Һ������ʹƷ����ɫ����������ɫ����Һ������ߵ�ʵ�鷽��_________________________________________________________________
___________________________________________________________________��
(3)�ٳ�ʵ��˵��X�������Ա����ʵ�������ǿ���û�ѧ����ʽ��ʾ����
___________________________________________________________________��
(4)���壨CN��2��X��ѧ�������ƣ�Ҳ����H2��Ӧ����HCN����ˮ��Һ��һ���ᣩ��
��HCN�����к���4�����ۼ�����ṹʽ��__________________________________��
��KCN��Һ�Լ��ԣ�ԭ���ǣ������ӷ���ʽ��ʾ��_____________________________��
(5)���������£�������Z��ij����M����MCR3��CΪ̼Ԫ�أ���ȫ��Ӧ����CR2��MmRn(m��n��Ϊ������)����CR2����Ϊ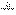 1g��MmRn����Ϊ
1g��MmRn����Ϊ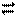 2g��M�����ԭ������Ϊa����MmRn��m:n��_____________(�ú�
2g��M�����ԭ������Ϊa����MmRn��m:n��_____________(�ú� ��a�Ĵ���ʽ��ʾ)��
��a�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com