
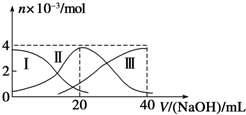
 �����£���20mL 0.2mol/L H2A��Һ�еμ�0.2mol/L NaOH��Һ���й��������ʵ����仯��ͼ�����Т����H2A�������HA-�������A2-����ʾ������ͼʾ�жϣ�����˵����ȷ���ǣ�������
�����£���20mL 0.2mol/L H2A��Һ�еμ�0.2mol/L NaOH��Һ���й��������ʵ����仯��ͼ�����Т����H2A�������HA-�������A2-����ʾ������ͼʾ�жϣ�����˵����ȷ���ǣ�������| A����V��NaOH��=20 mLʱ����Һ������Ũ�ȴ�С��ϵ��c��Na+����c��HA-����c��A2-����c��H+����c��OH-�� | B����Ũ�ȵ�NaOH��Һ��H2A��Һ��2��1��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮ�� | C��NaHA��Һ�У�c��OH-��+c��A2-��=c��H+��+c��H2A�� | D������������20mLNaOH��Һ��������Һ���ټ���ˮ�Ĺ����У�pH���ܼ��� |


 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
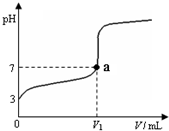 ��2011?�����ж�ģ�������£���20mL x mol?L-1 CH3COOH��Һ����μ�������ʵ���Ũ�ȵ�NaOH��Һ�����Һ��pH��NaOH��Һ�������V���ı仯��ϵ��ͼ��ʾ�������¶ȱ仯��������˵������ȷ���ǣ�������
��2011?�����ж�ģ�������£���20mL x mol?L-1 CH3COOH��Һ����μ�������ʵ���Ũ�ȵ�NaOH��Һ�����Һ��pH��NaOH��Һ�������V���ı仯��ϵ��ͼ��ʾ�������¶ȱ仯��������˵������ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
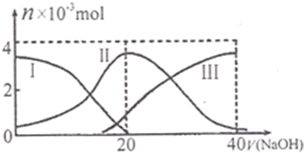
 ��֪������CH3COOH�ĵ���ƽ�ⳣ��ΪKa�������£���20mL 0.1mol?L-1 CH3COOH��Һ����μ���0.1mol?L-1NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯��������˵������ȷ���ǣ�������
��֪������CH3COOH�ĵ���ƽ�ⳣ��ΪKa�������£���20mL 0.1mol?L-1 CH3COOH��Һ����μ���0.1mol?L-1NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯��������˵������ȷ���ǣ�������| A��a���ʾ����Һ����ˮ�������H+Ũ��Ϊ1.0��10-3mol?L-1 | ||
| B��b���ʾ����Һc��CH3COO-����c��Na+�� | ||
| C��c���ʾCH3COOH��NaOHǡ�÷�Ӧ��ȫ | ||
D��b��d���ʾ����Һ��
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
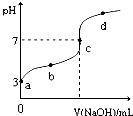
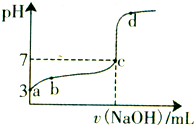 �����£���20mL 0.1moL/L CH3COOH��Һ����μ���0.1mol/L NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯��������˵������ȷ���ǣ�������
�����£���20mL 0.1moL/L CH3COOH��Һ����μ���0.1mol/L NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯��������˵������ȷ���ǣ�������| A��a���ʾ����Һ��c��OH-��=10-11mol/L | ||
B��a��b���ʾ����Һ��
| ||
| C��c���ʾCH3COOH��NaOHǡ����ȫ��Ӧc��CH3COOH��?c��OH-�� | ||
| D��d���ʾ����Һ��c��Na+����c��CH3COO-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
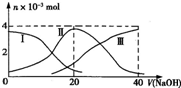 �����£���20mL 0.2mol?L-1 H2SO3����Һ�еμ�0.2mol?L-1 NaOH��Һ���й��������ʵ����仯������ͼ��ʾ��������I����H2SO3�������HS
�����£���20mL 0.2mol?L-1 H2SO3����Һ�еμ�0.2mol?L-1 NaOH��Һ���й��������ʵ����仯������ͼ��ʾ��������I����H2SO3�������HS| O | - 3 |
| O | 2- 3 |
| A����V��NaOH��=0ʱ����ˮ�������c��H+��=1.0��10-12?? | ||||
B����V��NaOH��=20 mLʱ��c��Na+����c��HS
| ||||
C����V��NaOH��=40 mLʱ2c��Na+��=c��S
| ||||
| D����V��NaOH��=40 mL�����μ�NaOH��Һ����Һ���¶Ȼ�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com