
�����а��ֲ���������
A��CH2===CH—CH===CH2
B��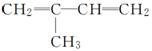
C��CH2===CH—CH===CH—CH3
D��
E��
F��CH��CH
G��CH3—C��CH
H��CH3—C��C—CH3
(1)��֪A��F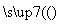
 ����Ҫ�ϳ�
����Ҫ�ϳ�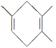 ����������ѡԭ����ȷ����________(�����)��
����������ѡԭ����ȷ����________(�����)��
��D��H����E��H����E��F����B��H����C��H
��D��G
(2)B��Br2��CCl4��Һ������Ӧʱ�������ɶ��ֲ��д����Щ����Ľṹ��ʽ��______________________��____________________��________________________��__________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��FeCl3��Һʴ��ͭ�������·��Ĺ����У���Һ��������Դ���յĹ��̼������£�
��.���Һ��Ͷ�������м����ַ�Ӧ�������������Һ��
��.����Һ�м���һ����ʯ��ˮ��������ҺpH��ͬʱ���������Ŀ�����
(1)FeCl3ʴ��ͭ����Ӧ�����ӷ���ʽΪ______________________________
_______________________________________________________________��
(2)���̢������м����Ҫ������__________________________________��
����õ��Ĺ�����Ҫ�ɷ���_______________________________________��
�ӹ����з����ͭ����õķ�����_________________________________��
(3)���̢��з�����Ӧ�Ļ�ѧ����ʽΪ_______________________________
________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�ͬ���칹����Ŀ����������ȷ���� (����)
A���ױ������ϵ�һ����ԭ�ӱ���3��̼ԭ�ӵ����ȡ�������ò�����6��
B���� ��Ϊͬ���칹��ķ����廯������6��
��Ϊͬ���칹��ķ����廯������6��
C������5��̼ԭ�ӵ�ij������������һ��ȡ���������3��
D���ƵĽṹ��ʽΪ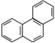 ���������ᷴӦ�������� 5��һ����ȡ����
���������ᷴӦ�������� 5��һ����ȡ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й����л����˵���в���ȷ����(����)
A����������춡����ۡ��е㲻��ͬ
B����ϩ��������������е�����ԭ�Ӷ���ͬһƽ����
C��C4H9Br��ͬ���칹����4��
D����ϩ�ͼ���������Ը��������Һ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ҫ����д����ʽ��
(1)��֪��Ũ���������������Һ�У���������ɫ���壬����Һ���Ϻ�ɫ��ȥ����һ������ԭ��Ӧ����ϵ�У�����KCl��Cl2��ŨH2SO4��H2O��KMnO4��MnSO4��K2SO4�������ʡ�
��д��һ�����������������ʵ�������ԭ��Ӧ����ʽ������ƽ________________________________________________________________________
________________________________________________________________________��
���ڷ�Ӧ�����Һ�м���NaBiO3(��������ˮ)����Һ�ֱ�Ϊ�Ϻ�ɫ��BiO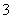 ��Ӧ���Ϊ��ɫ��Bi3����д����ʵ�����漰��Ӧ�����ӷ���ʽ________________________________________________________________________��
��Ӧ���Ϊ��ɫ��Bi3����д����ʵ�����漰��Ӧ�����ӷ���ʽ________________________________________________________________________��
(2)��6�۸��Ķ��Ժ�ǿ����ȡ�췯�ƺ�ķ�ˮ�к��е�Cr2O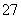 ���������̷���ȥ����÷�Ӧ�����Һ�к�Cr3����Fe2����Fe3����H���������ӡ�д���÷�Ӧ�����ӷ���ʽ________________________________________________________________________
���������̷���ȥ����÷�Ӧ�����Һ�к�Cr3����Fe2����Fe3����H���������ӡ�д���÷�Ӧ�����ӷ���ʽ________________________________________________________________________
________________________________________________________________________��
(3)KMnO4����������pH�ļ�С�����������Խ����л�ԭ������Mn2���������Ի���Խ����л�ԭ������Ҫ��MnO2��������ϩ(C2HCl3)�ǵ���ˮ�л���Ⱦ�����Ҫ�ɷ֣��о���ʾ�ڵ���ˮ�м���KMnO4��Һ�ɽ����е�������ϩ��ȥ����������ֻ��CO2��д����Ӧ�Ļ�ѧ����ʽ________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijС��ͬѧΪ̽��H2O2��H2SO3��Br2��������ǿ�����������ʵ��(�г���������ȥ��װ�õ��������Ѽ���)��
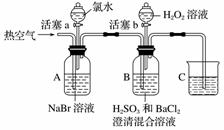
ʵ���¼���£�
| ʵ����� | ʵ������ | |
| �� | ����a���μ���ˮ�� �رջ���a | A����Һ��Ϊ����ɫ |
| �� | �����ȿ��� | A�к���ɫ���Ա�dz��B�������ݣ�����������ɫ���������Һ��ɫ�����Ա仯 |
| �� | ֹͣ�������������b����μ���H2O2��Һ | ��ʼʱ��ɫ�����Ա仯�������μ�H2O2��Һ��һ��ʱ����Һ��ɺ���ɫ |
��ش��������⣺
(1)A�з�Ӧ�����ӷ���ʽ��________________________________________________________________________��
(2)ʵ����������ȿ�����Ŀ����________________________________________________________________________��
(3)װ��C��������__________��C��ʢ�ŵ�ҩƷ��______________________��
(4)ʵ��������Һ��ɺ���ɫ�����Ӧ�����ӷ���ʽ��________________________________________________________________________��
(5)������ʵ��ó��Ľ�����________________________________________________________________________��
(6)ʵ�鷴˼��
����ͬѧ��Ϊʵ�����������ȿ����������(5)�н��۵ĵó�������Ϊ�Ƿ���ţ�������________________________________________________________________________��
��ʵ�������ʼʱ��ɫ�����Ա仯��ԭ����(д��һ������)________________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�δ�������ҽѧ���δȵġ�ϴԩ��¼�����������鶾�ļ��أ��������鶾���漰�Ļ�ѧ��Ӧ��4Ag��2H2S��O2===2X��2H2O������˵����ȷ����(����)
A��X�Ļ�ѧʽΪAgS
B�������鶾ʱ������������ʧȥ����
C����Ӧ��Ag��H2S���ǻ�ԭ��
D��ÿ����1 mol X����Ӧת��2 mol����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��A��B��C��D��E��F����Ԫ�أ���֪��
������λ��������ͬ�����ڣ��˵������������
��EԪ�صĵ��������ݼ��±�(kJ��mol��1)��
| I1 | I2 | I3 | I4 | �� |
| 496 | 4 562 | 6 912 | 9 540 | �� |
��B��Fͬ���塣
��A��E�ֱ�����D��ԭ�Ӹ�����1��1��2��1�γɻ����
��B��C�ֱ�����D��ԭ�Ӹ�����1��1��1��2�γɻ����
(1)д��ֻ����A��B��D��E����Ԫ�ص�������ˮ�εĻ�ѧʽ______________��______________��
(2)B2A2�����д���_________���Ҽ���_________���м���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com