
| A�� | pH��ͬ��CH3COONa��Һ��C6H5ONa��Һ��Na2CO3��Һ��NaOH��Һ��c��CH3COONa����c��C6H5ONa ����c��Na2CO3����c��NaOH �� | |
| B�� | �ڳ����£�10 mL 0.02 mol•L-1HCl��Һ��10 mL 0.02 mol•L-1 Ba��OH��2��Һ��ֻ�ϣ�����Ϻ���Һ�����Ϊ20 mL������Һ��pH=10 | |
| C�� | ��pH=3��һԪ����HA��pH=11��NaOH��Һ��ֻ�Ϻ�һ���У�c��OH-����c��H+����c��Na+����c��A-�� | |
| D�� | ��0.2 mol•L-1��������0.1 mol•L-1��NaAlO2��Һ�������ϣ�����Һ������Ũ����С�����˳��Ϊ��c��OH-����c��Al3+����c��H+����c��Na+����c��Cl-�� |
���� A��NaOH��ǿ����Һ��pH��ͬʱ��Ũ����С��pH��ͬ�����Σ��������ˮ��̶�Խ����Ũ��ԽС��
B�������£�n��HCl��=0.02mol/L��0.01L=0.0002mol��n[Ba��OH��2]=0.02mol/L��0.01L=0.0002mol������һԪ�ᡢ���Ƕ�Ԫ�������������ʣ�࣬��Һ�ʼ��ԣ������Һ��c��OH-��=$\frac{0.0002mol����2-1��}{0.02L}$=0.01mol/L��c��H+��=$\frac{{K}_{w}}{c��O{H}^{-}��}$��
C���������ᡢ����ǿ�pH=3������HA��pH=11��ǿ�c��HA����c��NaOH������Һ���δ֪�������Һ����Ϊ���ԡ����Ի���ԣ�
D����������������1L����n��HCl��=0.2mol��n��NaAlO2��=0.1mol��n��HCl����n��NaAlO2��=0.2mol��0.1mol=2��1�������ķ�ӦΪH2O+NaAlO2+HCl=NaCl+Al��OH��3����Al��OH��3+3HCl=AlCl3+3H2O��NaAlO2��ȫ��Ӧ���ɳ�����Ҫ0.1molHCl������Alԭ���غ�֪����0.1molAl��OH��3����ʣ��0.1molHCl��0.1molHCl�ܽ�Al��OH��3����AlCl3��������Һ�е�������AlCl3��NaCl������AlCl3�����ʵ���Ϊ0.033mol��
��� �⣺A��NaOH��ǿ����Һ��pH��ͬʱ��Ũ����С��pH��ͬ�����Σ��������ˮ��̶�Խ����Ũ��ԽС���������ˮ��̶�CO32-��C6H5O-��CH3COO-����pH��ͬ���⼸������Ũ�ȴ�С˳����c��CH3COONa����c��C6H5ONa ����c��Na2CO3����c��NaOH ������A��ȷ��
B�������£�n��HCl��=0.02mol/L��0.01L=0.0002mol��n[Ba��OH��2]=0.02mol/L��0.01L=0.0002mol������һԪ�ᡢ���Ƕ�Ԫ�������������ʣ�࣬��Һ�ʼ��ԣ������Һ��c��OH-��=$\frac{0.0002mol����2-1��}{0.02L}$=0.01mol/L��c��H+��=$\frac{{K}_{w}}{c��O{H}^{-}��}$=$\frac{1{0}^{-14}}{0.01}$mol/L=10-12 mol/L����Һ��pH=12����B����
C���������ᡢ����ǿ�pH=3������HA��pH=11��ǿ�c��HA����c��NaOH������Һ���δ֪�������Һ����Ϊ���ԡ����Ի���ԣ�������Һ������Ũ�Ȳ�һ����c��OH-����c��H+����c��Na+����c��A-������C����
D����������������1L����n��HCl��=0.2mol��n��NaAlO2��=0.1mol��n��HCl����n��NaAlO2��=0.2mol��0.1mol=2��1�������ķ�ӦΪH2O+NaAlO2+HCl=NaCl+Al��OH��3����Al��OH��3+3HCl=AlCl3+3H2O��NaAlO2��ȫ��Ӧ���ɳ�����Ҫ0.1molHCl������Alԭ���غ�֪����0.1molAl��OH��3����ʣ��0.1molHCl��0.1molHCl�ܽ�Al��OH��3����AlCl3��������Һ�е�������AlCl3��NaCl������AlCl3�����ʵ���Ϊ0.033mol��������ˮ���ʹ��Һ�����ԣ����������غ�֪��c��Cl-����������c��Na+����������ˮ�ˮ��̶Ƚ�С��������Һ������Ũ�ȴ�С˳����c��OH-����c��H+����c��Al3+����c��Na+����c��Cl-������D����
��ѡA��
���� ���⿼������Ũ�ȴ�С�Ƚϣ�Ϊ��Ƶ���㣬��ȷ��Һ�����ʼ��������ǽⱾ��ؼ������ؿ���ѧ�������жϼ������������ѵ���Dѡ����㣬ע��D�з����ķ�Ӧ����Ŀ�Ѷ��еȣ�


 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
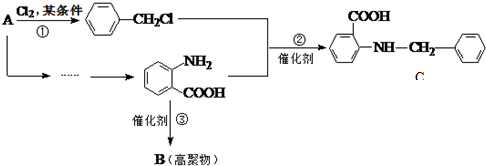
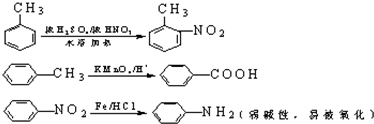
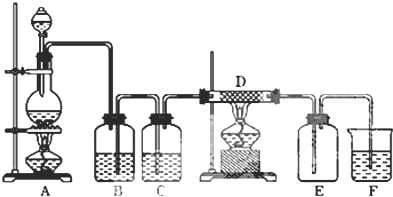
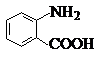 �й����ŵ����ư������Ȼ���
�й����ŵ����ư������Ȼ���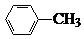 +Cl2$\stackrel{��}{��}$
+Cl2$\stackrel{��}{��}$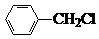 +HCl��
+HCl��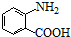 ��һ��̼��ͬϵ�����������������D��ͬ���칹�干��19�֣�д��һ�����������Һ�4�ֲ�ͬ��ԭ�ӵ�ͬ���칹��Ľṹ��ʽ
��һ��̼��ͬϵ�����������������D��ͬ���칹�干��19�֣�д��һ�����������Һ�4�ֲ�ͬ��ԭ�ӵ�ͬ���칹��Ľṹ��ʽ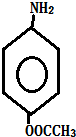 �ȣ�
�ȣ� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����Һ��c��K+����c��NO3-����c��Ag+����c��Cl-����c��I-�� | |
| B�� | �����Һ��c��K+����c��NO3-����c��Cl-����c��Ag+����c��I-�� | |
| C�� | ����AgNO3��Һʱ��������AgCl���� | |
| D�� | �����Һ��$\frac{c��C{l}^{-}��}{c��{I}^{-}��}$ԼΪ1.03��10-3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2O2����Ԫ�صĻ��ϼ���-2 | B�� | �Ҵ��ķ���ʽ��CH3CH2OH | ||
| C�� | 16S�Ľṹʾ��ͼ�� | D�� | ����ĽṹʽΪ��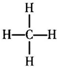 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
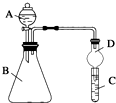 ijͬѧΪ̽��Ԫ�����ڱ���Ԫ�����ʵĵݱ���ɣ����������ϵ��ʵ�飮
ijͬѧΪ̽��Ԫ�����ڱ���Ԫ�����ʵĵݱ���ɣ����������ϵ��ʵ�飮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
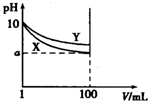 �����£�pH=10��X��Y���ּ���Һ��1mL���ֱ�ϡ����100mL����pH����Һ�����V���Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
�����£�pH=10��X��Y���ּ���Һ��1mL���ֱ�ϡ����100mL����pH����Һ�����V���Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | X��Y���ּ���Һ�����ʵ����ʵ���Ũ��һ����� | |
| B�� | ϡ�ͺ�X��Һ�ļ��Ա�Y��Һ�ļ���ǿ | |
| C�� | �ֱ���ȫ�к�X��Y�����ּ���Һʱ������ͬŨ������ �����Vx��Vy | |
| D�� | ��8��a��10����X��Y�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
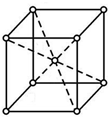 X��Y��Z��W����Ԫ�������ڱ�ǰ������Ԫ�أ�XԪ��ԭ�Ӻ�����16�ֲ�ͬ�˶�״̬�ĵ��ӣ�Y��ԭ��������X��1��Zԭ�ӵ�M�ܲ�����4��δ�ɶԵ��ӣ�W�ļ۲�����Ų�ʽΪndn+5��n+1��sn-1��
X��Y��Z��W����Ԫ�������ڱ�ǰ������Ԫ�أ�XԪ��ԭ�Ӻ�����16�ֲ�ͬ�˶�״̬�ĵ��ӣ�Y��ԭ��������X��1��Zԭ�ӵ�M�ܲ�����4��δ�ɶԵ��ӣ�W�ļ۲�����Ų�ʽΪndn+5��n+1��sn-1�� ��WԪ�ص�����Ϊ����
��WԪ�ص�����Ϊ�����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com