
| �� |
| �� |
| �� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ijѧ���������ͼ��ʾ��װ�ý���ʵ�飬��װ������װ�Լ���ʵ�������ʵ���������£�
ijѧ���������ͼ��ʾ��װ�ý���ʵ�飬��װ������װ�Լ���ʵ�������ʵ���������£�| �� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| O | 2- 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����Ǽ�����Ҫ�Ļ���ԭ�ϣ���ҵ�Ͻ�������NO2���ܱ���������ˮ��η���ѭ�������Ʊ����ᣮ
�����Ǽ�����Ҫ�Ļ���ԭ�ϣ���ҵ�Ͻ�������NO2���ܱ���������ˮ��η���ѭ�������Ʊ����ᣮ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ��ԭ�ر�����ѧ��һ�������¿���ѧ�Ծ����������� ���ͣ�ʵ����
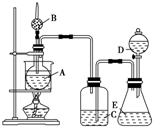
(11��)Ϊ֤���й����ʵ����ʣ�ijѧ���������ͼ��ʾ��װ�á�������C��ʹҺ��A�����H�ϵĹ���B����ʱ���������������������D������Ϩ�𣻹ر�C ��Eʱ����G������ȼ�ø�����
��1�����жϸ�������ʢ�ŵ����ʵĻ�ѧʽ��
A �� B ��F ��
��2���û�ѧ��Ӧ����ʽ��ʾ�����йط�Ӧ�������������ȼ�գ���
,
,
.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��߿���ѧһ�ָ�ϰ�����������ר���ۺϲ��ԣ��ս̰棩 ���ͣ������
(8��)(2011���������ݵ���)Ϊȷ��C6H5OH��H2CO3��CH3COOH������ǿ����ijѧ���������ͼ��ʾ��װ�ã�һ��ʵ�鼴�ɴﵽĿ��(����ѡ������������)��
��ݴ�ʵ��ش�
(1)��ƿ��װij������������Һ����Һ©������ʢ�Լ�ӦΪ________��
(2)װ��B��ʢ���Լ���________����������______________________________��
(3)װ��C��ʢ���Լ���________��C�з�Ӧ�����ӷ���ʽ��______________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com