
���;�ˮ���������(K2FeO4)Ϊ����ɫ���壬������ˮ�������Ի�������Һ���ֽ⣬�ڼ�����Һ���ȶ���
��ҵ���Ʊ�K2FeO4�ij��÷��������֡�
��������������������
����������ͼ��ʾ��
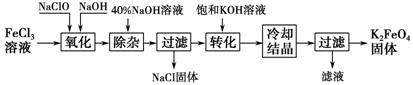
(1)��ɡ������������з�Ӧ�Ļ�ѧ����ʽ��
FeCl3��____NaOH��____NaClO��____Na2FeO4��____��____��������������____(�ѧʽ)��
(2)��ת���������з�����Ӧ�Ļ�ѧ����ʽΪ________________________________________________________________________
________________________________________________________________________��
(3)�������յõ��ĸ�����س��������ʣ������ؽᾧ���ᴿ�������ǣ����ֲ�Ʒ��________________�ܽ⣬Ȼ��________________��
������ⷨ��
����Ϊ�����������������Һ��Ȼ��������Һ�м���KOH��
(4)���ʱ����������Ӧ����FeO���õ缫��Ӧ����ʽΪ________________________________________________________________________��
������(1)��Ӧ��NaClO������������ԭ������NaCl������ԭ���غ㣬��֪��Ӧʽ����Ҫ����NaCl��H2O�����ݻ��ϼ���������ƽ��ѧ����ʽΪ2FeCl3��10 NaOH��3NaClO===2Na2FeO4��9NaCl��5H2O��(2)����(1)�з�Ӧ�Ļ�ѧ����ʽ�͡�ת���������յõ��IJ����֪��ת�����������ڼ���KOH��Һ��Na2FeO4ת��Ϊ�ܽ�ȸ�С��K2FeO4��(3)��ΪK2FeO4�����Ի�������Һ���ֽܷ⣬������Ҫ��K2FeO4�ֲ�Ʒ��ϡKOH��Һ���ܽ⣬Ȼ����뱥��KOH��Һ����ȴ�ᾧ��(4)���ʱ��������ǿ���������±�����ΪFeO��Fe��8OH����6e��===FeO��4H2O��
�𰸡�(1)2��10��3��2��9��NaCl��5��H2O��NaClO��(2)Na2FeO4��2KOH===K2FeO4��2NaOH��(3)ϡKOH��Һ�����뱥��KOH��Һ����ȴ�ᾧ��(4)Fe��8OH����6e��===FeO��4H2O



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijС��ͬѧ��������ͼװ�õ���������Һ��
��ȡ������������������������ء�
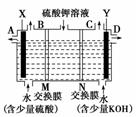
(1)X�����Դ��________(���������)��������������________(ѡ�A������B������C����D��)�ڵ�����
(2)���ӽ���Ĥֻ����һ������ͨ������MΪ________(������ӡ��������ӡ�����ͬ)����Ĥ��NΪ________����Ĥ��
(3)�����Ƶõ�����������������������Һ���Ϊ����ȼ�ϵ��(ʯīΪ�缫)�����ظ����ĵ缫��ӦʽΪ________________________________________________________
________________________________________________________________________��
(4)���ڱ�״���£��Ƶ�11.2 L�����������������������________��ת�Ƶĵ�����Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ۺ���ͼ�жϣ�������������ȷ����
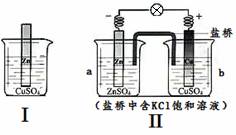
A. ��ķ�Ӧԭ������Zn + Cu2+ = Zn2+ + Cu
B. ���о��е���ת�ƣ����ǰѻ�ѧ��ת��Ϊ��������
C. ���ŷ�Ӧ�Ľ��У�����CuSO4��Һ��ɫ��������dz
D. ȡa����Һ��������Ba(NO3)2��Һ�����˺�����Һ�м�AgNO3��Һ���г�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���÷���˿������ͭ��Һ(����������)�ͱ��л�����Ⱦ�ķ�
ͭ���Ʊ�����ͭ���塣�����������£�
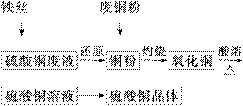 |
�Իش��������⣺
(1)��˿��Ͷ������ͭ��Һǰ����ϡH2SO4���д��������ܷ�����Ӧ�����ӷ���ʽ��_____________________��
(2)��ͭ���뻹ԭ����ͭ�ۻ�����գ����鷢�����պ�õ�����CuO������Cu�Ļ���ԭ������ǣ�
�����ղ����Cuδ����ȫ������
��CuO����ԭ����ԭ��������________���÷�Ӧ�Ļ�ѧ����ʽ��______________
________________________________________________________________________��
(3)Ϊ��ʹ���պ������ͭ����������ܣ��ڼ���ϡH2SO4��ͬʱ����ͨ��O2��ͨ��O2��Ŀ����(�û�ѧ��Ӧ����ʽ��ʾ)____________________��
(4)ֱ��������ͭ������м���Ũ���Ტ���Ƚ������ܣ�Ҳ�ɴﵽ������ܵ�Ŀ�ģ���ʵ�ʲ����н���ʹ�ã�ԭ�������__________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ѧ����ʵ�������������ȷ���� (����)��
A����������Ͷ�뵽һ������ϡ�����У���ַ�Ӧ��ȡ�ϲ���Һ���Թ��У��μ�KSCN��Һ����Һ��Ѫ��ɫ
B���Ʊ�����������ʱ��������������Һ�еμ�����������Һ���ӱ߽��裬�����Ƶð�ɫ������������
C�������ש�е��������ɷ֣����ש��ĩ�м������ᣬ��ַ�Ӧ��ȡ�ϲ���Һ���Թ��У��μ�KSCN��Һ2��3�μ���
D����CuSO4��Һ�е������NaOH��Һ��ַ�Ӧ�����Һ�嵹���������м������һ�ᣬȻ����ȴ�����ˣ���ֽ�ϵ�����Ϊ����ɫ���塱
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڳ�ѹ��500 �������£������ʵ�����Ag2O��Fe(OH)3��NH4HCO3��NaHCO3����ȫ�ֽ⣬���������������ΪV1��V2��V3��V4�������С˳����ȷ����(����)
A��V3>V2>V4>V1���� B��V3>V4>V2>V1
C��V3>V2>V1>V4���� D��V2>V3>V1>V4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮϡ�ͣ��ڶ�ͼ�л���pHֵ�ı仯ͼ��
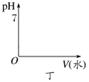
��ˮϡ����ͬ�ı�����________��pH��
��ˮϡ�͵���ͬ��pH��________�����ˮ�ࡣ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijС��ͬѧ��̼��Ϊ�缫���CuCl2��Һʱ����������̼���ϳ����к�ɫ���������⣬����������ɫ����������Ϊ̽������̼���ϵIJ��ͬѧ�����������ʵ����̡�
(1)�Ա�ʵ��(��̼��Ϊ�缫���������Һ)
| �������Һ | ������������ | |
| ʵ��1 | CuSO4��Һ | ��ɫ���� |
| ʵ��2 | CuSO4��NaCl�Ļ����Һ | ��ɫ�����������ɫ���� |
(2)�������
�ٺ�ɫ����һ����ͭ����������Cu2O��
�ڰ�ɫ����Ϊͭ�Ļ�����仯ѧʽ����Ϊ________��
(3)ʵ����֤
ȡ���CuCl2��Һ�������̼����ϴ�ӡ������������װ�ý���ʵ�飬��֤�������
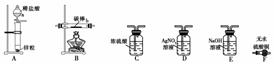
����a������Ϊ________����װ�ô������ҵ�����˳��Ϊ________��
(4)�۲����ó�����
ʵ�������̼���ϵİ�ɫ���ʱ�Ϊ��ɫ��F�����ʲ���ɫ��D�г��ְ�ɫ������
��������д��װ��B�з�����Ӧ�Ļ�ѧ����ʽ��
________________________________________________________________________��
(5)��������
����ϴȥ̼���ϵĺ�ɫ�Ͱ�ɫ���ʣ��ɽ�̼������ϡ�����У����ɫ������ʧ�����ӷ���ʽΪ______________________����ɫ������ʧ�����ӷ���ʽΪ_________________
______________________��
��ʵ������У���װ��B�еĿ���û���ž��Ϳ�ʼ���ȣ����ܶ�ʵ����ɵ�Ӱ����________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com