


 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����ͼ��ʾ������ת����ϵ�У�A�dz����ļ�����̬�⻯�B����ʹ�����ǵ�ľ����ȼ����ɫ��ζ���壬E����Է���������D��17��G�����ڽ������˳�������Ϻ�ɫ�������ʣ������ַ�Ӧ��������û��ȫ���г�����Ӧ����δ�г�����ش��������⣺
����ͼ��ʾ������ת����ϵ�У�A�dz����ļ�����̬�⻯�B����ʹ�����ǵ�ľ����ȼ����ɫ��ζ���壬E����Է���������D��17��G�����ڽ������˳�������Ϻ�ɫ�������ʣ������ַ�Ӧ��������û��ȫ���г�����Ӧ����δ�г�����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������λ�ڶ����ڵ��������ڡ���������ķǽ���Ԫ��X��Y����֪��Ԫ������������ˮ�����Ϊǿ�ᣮ������ͼת����ϵ����Ӧ���������ֲ�������ȥ�����ش��������⣺
������λ�ڶ����ڵ��������ڡ���������ķǽ���Ԫ��X��Y����֪��Ԫ������������ˮ�����Ϊǿ�ᣮ������ͼת����ϵ����Ӧ���������ֲ�������ȥ�����ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
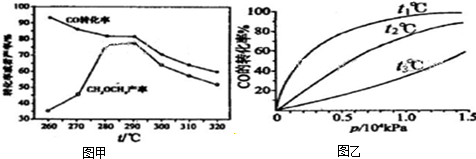
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ͼ�У����缫�Ϸ����ĵ缫��ӦΪ��a����Cu2++2e-=Cu��b����Fe-2eһ=Fe2+������˵���в���ȷ���ǣ�������
ͼ�У����缫�Ϸ����ĵ缫��ӦΪ��a����Cu2++2e-=Cu��b����Fe-2eһ=Fe2+������˵���в���ȷ���ǣ�������| A����װ�ÿ����ǵ��� |
| B��a���Ϸ������ǻ�ԭ��Ӧ |
| C��a��b��������ͬ�ֵ缫���� |
| D����װ�ù���ʱ����Һ�е���������b���ƶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| A��483.6 kJ |
| B��88 kJ |
| C��285.8 kJ |
| D��44 kJ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com