
ij»ÆѧŠĖȤŠ”×éŅŌľĢæŗĶÅØĻõĖįĪŖĘšŹ¼ŌĮĻ£¬Ģ½¾æŅ»Ńõ»ÆµŖÓė¹żŃõ»ÆÄĘ·“Ó¦ÖʱøŃĒĻõĖįÄĘ”£Éč¼Ę×°ÖĆČēĻĀ(ŗöĀŌ×°ÖĆÖŠæÕĘųµÄÓ°Ļģ)£¬Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ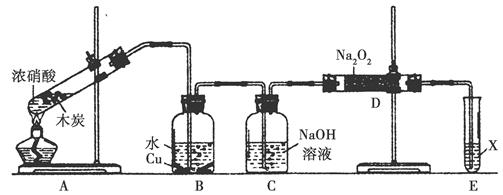
£Ø1£©×é×°ŗĆŅĒĘ÷ŗ󣬱ŲŠė½ųŠŠµÄŅ»Ļī²Ł×÷ŹĒ_________________”£
£Ø2£©×°ÖĆAµÄŹŌ¹ÜÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ_______________”£
£Ø3£©ĶĘ²āBÖŠæÉŅŌ¹Ū²ģµ½µÄÖ÷ŅŖĻÖĻóŹĒ________£»C×°ÖƵÄ×÷ÓĆŹĒ________”£
£Ø4£©×°ÖĆDÖŠ³żÉś³ÉNaNO2Ķā£¬»¹ÓŠĮķŅ»ÖÖ¹ĢĢ¬ĪļÖŹY£¬YµÄ»ÆѧŹ½ŹĒ________£»æÉŅŌĶعżŹŹµ±øĽų£¬²»²śÉśYĪļÖŹ£¬ĒėÄćĢį³öøĽų·½·Ø£ŗ______________________”£
£Ø5£©ŅŃÖŖ£ŗŃĒĻõĖįŹĒČõĖį£¬²»ĪČ¶Ø£¬ŹŅĪĀĻĀ“ęŌŚ·“Ó¦3HNO2=HNO3£«2NO”ü£«H2O£»ŌŚĖįŠŌČÜŅŗÖŠ£¬NO2-æɽ«MnO4-»¹ŌĪŖMn2£«ĒŅĪŽĘųĢåÉś³É”£
¢ŁŠ“³ö¼ģŃéDÖŠ²śĪļŹĒŃĒĻõĖįÄʵķ½·Ø£ŗ_________________£»
¢ŚE×°ÖĆÖŠŹŌ¼ĮXæÉŅŌŹĒ________”£
| A£®Ļ”ĮņĖį | B£®ĖįŠŌøßĆĢĖį¼ŲČÜŅŗ |
| C£®Ļ”ĻõĖį | D£®Ė® |
£Ø1£©¼ģ²é×°ÖƵÄĘųĆÜŠŌ
£Ø2£©C£«4HNO3(ÅØ)  CO2£«4NO2”ü£«2H2O
CO2£«4NO2”ü£«2H2O
£Ø3£©Ķʬ֚½„Čܽā£¬ČÜŅŗÖš½„±äĄ¶£¬²śÉśĪŽÉ«ĘųÅŻ””³żČ„NOÖŠ»ģÓŠµÄCO2
£Ø4£©NaOH””ÓĆ×°ÓŠ¼īŹÆ»ŅµÄøÉŌļ¹Ü“śĢęC×°ÖĆ
£Ø5£©¢Ł½«Éś³ÉĪļÖĆÓŚŹŌ¹ÜÖŠ£¬¼ÓČėĻ”ĮņĖį£¬Čō²śÉśĪŽÉ«ĘųĢå²¢ŌŚŅŗĆęÉĻ·½±äĪŖŗģ×ŲÉ«£¬ŌņDÖŠ²śĪļŹĒŃĒĻõĖįÄĘ(»ņ½«Éś³ÉĪļÖĆÓŚŹŌ¹ÜÖŠ£¬¼ÓČėĖįŠŌKMnO4ČÜŅŗ£¬ČōČÜŅŗ×ĻÉ«ĶŹČ„£¬ŌņDÖŠ²śĪļŹĒŃĒĻõĖįÄĘ)””¢ŚB
½āĪö


 ±øÕ½ÖŠæ¼ŗ®¼ŁĻµĮŠ“š°ø
±øÕ½ÖŠæ¼ŗ®¼ŁĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ijŠĖȤŠ”×éÉč¼Ę³öĻĀĶ¼ĖłŹ¾×°ÖĆĄ“øĽų½Ģ²ÄÖŠ”°ĶÓėĻõĖį·“Ó¦”±ŹµŃ飬ŅŌĢ½¾æ»ÆѧŹµŃéµÄĀĢÉ«»Æ”£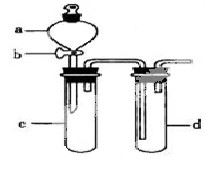
£Ø1£©ŹµŃéĒ°£¬¹Ų±Õ»īČūb£¬ŹŌ¹ÜdÖŠ¼ÓĖ®ÖĮ½žĆ»³¤µ¼¹ÜæŚ£¬Čū½ōŹŌ¹ÜcŗĶdµÄ½ŗČū£¬¼ÓČČc£¬ĘäÄæµÄŹĒ__________”£
£Ø2£©ŌŚdÖŠ¼ÓŹŹĮæNaOHČÜŅŗ£¬cÖŠ·ÅŅ»Š”æéĶʬ£¬ÓÉ·ÖŅŗĀ©¶·aĻņcÖŠ¼ÓČė2mLÅØĻõĖį£¬cÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ______________________”£
ŌŁÓÉaĻņcÖŠ¼Ó2mLÕōĮóĖ®£¬cÖŠµÄŹµŃéĻÖĻóŹĒ_____________”£
£Ø3£©ĻĀ±ķŹĒÖĘČ”ĻõĖįĶµÄČżÖÖ·½°ø£¬ÄÜĢåĻÖĀĢÉ«»ÆѧĄķÄīµÄ×ī¼Ń·½°øŹĒ_____£¬ĄķÓÉŹĒ_______”£
| ·½°ø | ·“Ó¦Īļ |
| ¼× | Cu”¢ÅØĻõĖį |
| ŅŅ | Cu”¢Ļ”ĻõĖį |
| ±ū | Cu”¢O2”¢Ļ”ĻõĖį |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
¶žŃõ»ÆĆĢÓėÅØŃĪĖį»ģŗĻ¼ÓČȵƵ½ĀČĘų£¬ČēĶ¼ŹĒÖĘČ”²¢Ģ½¾æCl2»ÆѧŠŌÖŹµÄ×°ÖĆĶ¼”£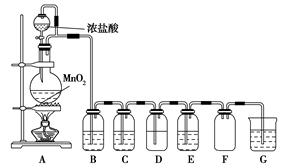
(1)Ō²µ×ÉÕĘæÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ”£
(2)ČōŅŖµĆµ½øÉŌļ“æ¾»µÄĘųĢ壬B”¢CÖŠÓ¦·Ö±šŹ¢·ÅµÄŹŌ¼ĮĪŖ ”¢ ”£
(3)EÖŠČō×°ÓŠFeCl2ČÜŅŗ£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ £¬EÖŠČō×°ÓŠµķ·Ūµā»Æ¼ŲČÜŅŗ£¬ÄܹŪ²ģµ½µÄŹµŃéĻÖĻóŹĒ ”£
(4)ŹµŃéÖŠ·¢ĻÖ£ŗÅØŃĪĖįÓėMnO2»ģŗĻ¼ÓČČÉś³ÉĀČĘų£¬Ļ”ŃĪĖįÓėMnO2»ģŗĻ¼ÓČČ²»Éś³ÉĀČĘų”£Õė¶ŌÉĻŹöĻÖĻóij»ÆѧŠĖȤŠ”×é¶Ō”°Ó°ĻģĀČĘųÉś³ÉµÄŌŅņ”±½ųŠŠĮĖĢÖĀŪ£¬²¢Éč¼ĘĮĖŅŌĻĀŹµŃé·½°ø£ŗ
a£®Ļ”ŃĪĖįµĪČėMnO2ÖŠ£¬Č»ŗóĶØČėHClĘųĢå¼ÓČČ
b£®Ļ”ŃĪĖįµĪČėMnO2ÖŠ£¬Č»ŗó¼ÓČėNaCl¹ĢĢå¼ÓČČ
c£®Ļ”ŃĪĖįµĪČėMnO2ÖŠ£¬Č»ŗó¼ÓČėÅØĮņĖį¼ÓČČ
d£®MnO2ÓėNaClµÄÅØČÜŅŗ»ģŗĻ¼ÓČČ
e£®ÅØĮņĖįÓėNaCl¹ĢĢ唢MnO2¹ĢĢå¹²ČČ
¢ŁŹµŃébµÄÄæµÄŹĒ £¬ŹµŃécµÄÄæµÄŹĒ ”£
¢ŚŹµŃéĻÖĻó£ŗa”¢c”¢eÓŠ»ĘĀĢÉ«ĘųĢåÉś³É£¬b”¢dƻӊ»ĘĀĢÉ«ĘųĢåÉś³É”£ÓÉ“ĖµĆ³öÓ°ĻģĀČĘųÉś³ÉµÄŌŅņŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ĻĀĶ¼¼×ŹĒæĪ±¾ÖŠŃéÖ¤ĶŗĶÅØĻõĖį·“Ó¦µÄ×°ÖĆ£¬ŅŅ”¢±ūŹĒŹ¦Éś¶ŌŃŻŹ¾ŹµŃéøĽųŗóµÄ×°ÖĆ£ŗ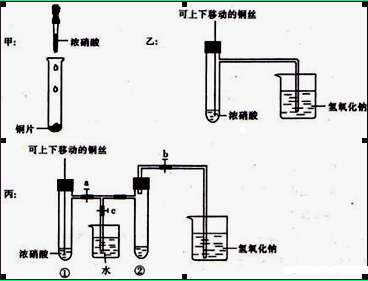
£Ø1£©¼×”¢ŅŅ”¢±ūČżøö×°ÖĆÖŠ¹²Ķ¬·¢ÉśµÄ»Æѧ·½³ĢŹ½ŹĒ ”£
£Ø2£©ŗĶ¼××°ÖĆĻą±Č£¬ŅŅ×°ÖƵÄÓŵćŹĒæÉŅŌæŲÖĘ·“Ó¦µÄæŖŹ¼ÓėĶ£Ö¹£¬»¹æÉŅŌ ”£
£Ø3£©ĪŖĮĖ½ųŅ»²½ŃéÖ¤NO2ŗĶĖ®µÄ·“Ó¦£¬µ±ĘųĢå³äĀś¢ŚŹŌ¹Üŗ󣬽«ĶĖæĢįĘšÓėČÜŅŗĶŃĄė”£ÓūŹ¹ÉÕ±ÖŠµÄ Ė®½ųČĖ¢ŚŹŌ¹Ü£¬Ó¦ČēŗĪ²Ł×÷?
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
¾§Ģå¹čŹĒŅ»ÖÖÖŲŅŖµÄ·Ē½šŹō²ÄĮĻ£¬Öʱø“æ¹čµÄÖ÷ŅŖ²½ÖčČēĻĀ£ŗ
¢ŁøßĪĀĻĀÓĆĢ¼»¹Ō¶žŃõ»Æ¹čÖĘµĆ“Ö¹č
¢Ś“Ö¹čÓėøÉŌļHClĘųĢå·“Ó¦ÖʵĆSiHCl3£ŗSi£«3HCl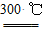 SiHCl3£«H2
SiHCl3£«H2
¢ŪSiHCl3Óė¹żĮæH2ŌŚ1 000”«1 100 ”ę·“Ó¦ÖʵƓæ¹č”£
ŅŃÖŖSiHCl3ÄÜÓėH2OĒæĮŅ·“Ó¦£¬ŌŚæÕĘųÖŠŅ××ŌČ¼”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)µŚ¢Ł²½Öʱø“Ö¹čµÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ_______________________________”£
(2)“Ö¹čÓėHCl·“Ó¦ĶźČ«ŗ󣬾ĄäÄżµĆµ½µÄSiHCl3(·Šµć£33 ”ę)ÖŠŗ¬ÓŠÉŁĮæSiCl4(·Šµć57.6 ”ę)ŗĶHCl(·Šµć£84.7 ”ę)£¬Ģį“æSiHCl3²ÉÓƵķ½·ØĪŖ________”£
(3)ÓĆSiHCl3Óė¹żĮæH2·“Ó¦Öʱø“æ¹čµÄ×°ÖĆČēĻĀĶ¼(ČČŌ“¼°¼Š³Ö×°ÖĆĀŌČ„)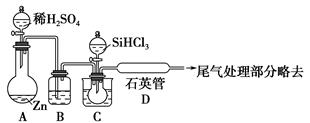
¢Ł×°ÖĆBÖŠµÄŹŌ¼ĮŹĒ________”£×°ÖĆCÖŠµÄÉÕĘæŠčŅŖ¼ÓČČ£¬ĘäÄæµÄŹĒ
________________________________________________________________ӣ
¢Ś·“Ó¦Ņ»¶ĪŹ±¼äŗó£¬×°ÖĆDÖŠ¹Ū²ģµ½µÄĻÖĻóŹĒ________£¬×°ÖĆD²»ÄܲÉÓĆĘÕĶز£Į§¹ÜµÄŌŅņŹĒ__________________________________”£
×°ÖĆDÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_______________________________”£
¢ŪĪŖ±£Ö¤Öʱø“æ¹čŹµŃéµÄ³É¹¦£¬²Ł×÷µÄ¹Ų¼üŹĒ¼ģ²éŹµŃé×°ÖƵÄĘųĆÜŠŌ£¬æŲÖĘŗĆ·“Ó¦ĪĀ¶ČŅŌ¼°__________________________________________________
¢ÜĪŖ¼ų¶Ø²śĘ·¹čÖŠŹĒ·ńŗ¬Ī¢ĮæĢśµ„ÖŹ£¬½«ŹŌŃłÓĆĻ”ŃĪĖįČܽā£¬Č”ÉĻ²ćĒåŅŗŗóŠčŌŁ¼ÓČėµÄŹŌ¼ĮŹĒ(ĢīŠ“×ÖÄø“śŗÅ)________”£
a£®µāĖ®””b£®ĀČĖ®””c£®NaOHČÜŅŗ””d£®KSCNČÜŅŗ
e£®Na2SO3ČÜŅŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ĄūÓĆ¼×ĶéÓėĀČĘų·¢ÉśČ”“ś·“Ó¦ÖĘČ”ø±²śĘ·ŃĪĖįµÄÉčĻėŌŚ¹¤ŅµÉĻŅŃ³ÉĪŖĻÖŹµ”£Ä³»ÆѧŠĖȤŠ”×éŌŚŹµŃéŹŅÖŠÄ£ÄāÉĻŹö¹ż³Ģ£¬ĘäÉč¼ĘµÄÄ£Äā×°ÖĆČēĻĀ£ŗ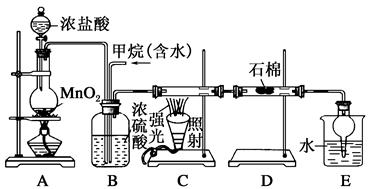
øł¾ŻÉč¼ĘŅŖĒó»Ų“š£ŗ
£Ø1£©B×°ÖĆÓŠČżÖÖ¹¦ÄÜ£ŗ¢ŁæŲÖĘĘųĮ÷ĖŁ¶Č£»¢Ś¾łŌČ»ģŗĻĘųĢ壻¢Ū______________”£
£Ø2£©ÉčV£ØCl2£©/V£ØCH4£©£½x£¬ČōĄķĀŪÉĻÓū»ńµĆ×ī¶ąµÄĀČ»ÆĒā£¬ŌņxÖµÓ¦________”£
£Ø3£©D×°ÖƵďÆĆŽÖŠ¾łŌČ»ģÓŠKI·ŪÄ©£¬Ęä×÷ÓĆŹĒ____________”£
£Ø4£©E×°ÖƵÄ×÷ÓĆŹĒ________£ØĢīŠņŗÅ£©”£
| A£®ŹÕ¼ÆĘųĢå | B£®ĪüŹÕĀČĘų |
| C£®·ĄÖ¹µ¹Īü | D£®ĪüŹÕĀČ»ÆĒā |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ij»ÆѧæĪĶā»ī¶ÆŠ”×éĶعżŹµŃéŃŠ¾æNO2µÄŠŌÖŹ”£
ŅŃÖŖ:2NO2+2NaOH NaNO3+NaNO2+H2O
NaNO3+NaNO2+H2O
ČĪĪń1:ĄūÓĆČēĶ¼ĖłŹ¾×°ÖĆĢ½¾æNO2ÄÜ·ń±»NH3»¹Ō(K1”¢K2ĪŖÖ¹Ė®¼Š,¼Š³Ö¹Ģ¶Ø×°ÖĆĀŌČ„)”£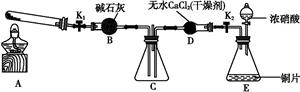
(1)E×°ÖĆÖŠÖĘČ”NO2·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”””£
(2)ČōNO2Äܹ»±»NH3»¹Ō,Ō¤ĘŚ¹Ū²ģµ½C×°ÖĆÖŠµÄĻÖĻóŹĒ”” ”£
(3)ŹµŃé¹ż³ĢÖŠ,Ī“ÄܹŪ²ģµ½C×°ÖĆÖŠµÄŌ¤ĘŚĻÖĻó”£øĆŠ”×éĶ¬Ń§“Ó·“Ó¦ŌĄķµÄ½Ē¶Č·ÖĪöĮĖŌŅņ,ČĻĪŖæÉÄÜŹĒ:
¢ŁNH3»¹ŌŠŌ½ĻČõ,²»Äܽ«NO2»¹Ō;
¢ŚŌŚ“ĖĢõ¼žĻĀ,NO2µÄ×Ŗ»ÆĀŹ¼«µĶ;
¢Ū”” ”£
(4)“ĖŹµŃé×°ÖĆ“ęŌŚŅ»øöĆ÷ĻŌµÄȱĻŻŹĒ ”£
ČĪĪń2:Ģ½¾æNO2ÄÜ·ńÓėNa2O2·¢ÉśŃõ»Æ»¹Ō·“Ó¦”£
(5)ŹµŃéĒ°,øĆŠ”×éĶ¬Ń§Ģį³öČżÖÖ¼ŁÉč”£
¼ŁÉč1:¶žÕß²»·“Ó¦;
¼ŁÉč2:NO2Äܱ»Na2O2Ńõ»Æ;
¼ŁÉč3:”””””””””””””””””””””” ”£
(6)ĪŖĮĖŃéÖ¤¼ŁÉč2,øĆŠ”×éĶ¬Ń§Ń”ÓĆČĪĪń1ÖŠµÄB”¢D”¢E×°ÖĆ,½«BÖŠµÄŅ©Ę·øü»»ĪŖNa2O2,ĮķŃ”F×°ÖĆ(ČēĶ¼ĖłŹ¾),ÖŲŠĀ×é×°,½ųŠŠŹµŃ锣
¢Ł×°ÖƵÄŗĻĄķĮ¬½ÓĖ³ŠņŹĒ”” ”£
¢ŚŹµŃé¹ż³ĢÖŠ,B×°ÖĆÖŠµ»ĘÉ«·ŪÄ©Öš½„±ä³É°×É«”£¾¼ģŃé,øĆ°×É«ĪļÖŹĪŖ“æ¾»Īļ,ĒŅĪŽĘäĖūĪļÖŹÉś³É”£ĶĘ²āB×°ÖĆÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ”””””””””””””””””””””£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
(1)ij»ÆѧŠĖȤŠ”×éµÄĶ¬Ń§½ųŠŠCl2”¢NH3µÄÖʱø¼°ŠŌÖŹ¼ģŃéµČŹµŃéµÄĮ÷³ĢŗĶ²æ·Ö×°ÖĆČēĻĀ£ŗ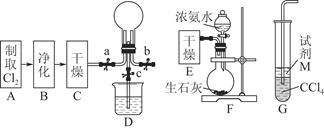
¢ŁĒėĄūÓĆA”¢G×°ÖĆÉč¼ĘŅ»øö¼ņµ„µÄŹµŃéŃéÖ¤Cl2”¢Fe3£«”¢I2µÄŃõ»ÆŠŌĒæČõĪŖCl2>Fe3£«>I2(ŹµŃéÖŠ²»¶ĻµŲŠ”ŠÄÕńµ“G×°ÖĆÖŠµÄŹŌ¹Ü)”£ĒėŠ“³öAÖŠ·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ______________________________________________£¬
ĒėŠ“³öŹŌ¼ĮMĪŖ________ČÜŅŗ£¬Ö¤Ć÷Ńõ»ÆŠŌĪŖCl2>Fe3£«>I2µÄŹµŃéĻÖĻóŹĒ________________________________________________________________________”£
¢ŚŅŃÖŖ3Cl2£«2NH3=6HCl£«N2£¬µ±DµÄÉÕĘæÖŠ³äĀś»ĘĀĢÉ«ĘųĢåŗ󣬹Ų±Õa”¢c“ņæŖb£¬DÖŠµÄĻÖĻóĪŖ»ĘĀĢÉ«ĘųĢåĻūŹ§£¬²śÉś°×ŃĢ£¬·“Ó¦Ņ»¶ĪŹ±¼äŗ󣬹Ų±Õb“ņæŖc£¬¹Ū²ģµ½µÄĻÖĻóĪŖ________________________________________________________________________”£
(2)ij·ĻĖ®ÖŠŗ¬ÓŠŅ»¶ØĮæµÄNa£«”¢SO32”Ŗ£¬æÉÄÜŗ¬ÓŠCO32”Ŗ£¬Ä³ŃŠ¾æŠ”×éÓū²ā¶ØĘäÖŠSO32”ŖµÄÅØ¶Č£¬Éč¼ĘČēĻĀŹµŃé·½°ø£ŗ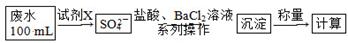
¢Ł“ÓĻĀĮŠŹŌ¼ĮÖŠŃ”ŌńŹŌ¼ĮXĪŖ________(ĢīŠņŗÅ)£»
A£®0.1 mol/L KMnO4(H2SO4Ėį»Æ)ČÜŅŗB£®0.5 mol/L NaOHČÜŅŗ
C£®ŠĀÖĘĀČĖ® D£®KIČÜŅŗ
¢Ś¼ÓČėŹŌ¼ĮXÉś³ÉSO42”ŖµÄĄė×Ó·½³ĢŹ½ĪŖ_______________________________________
¢ŪÖ¤Ć÷øĆ·ĻĖ®ÖŠŹĒ·ńŗ¬ÓŠCO32”ŖµÄŹµŃé·½°øĪŖ_________________________________________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
¹¤ŅµĪ²ĘųÖŠµŖŃõ»ÆĪļĶس£²ÉÓĆ°±“ß»ÆĪüŹÕ·Ø£¬ĘäŌĄķŹĒNH3ÓėNOxŌŚ“߻ƼĮ×÷ÓĆĻĀ·“Ӧɜ³ÉĪŽ¶¾µÄĪļÖŹ”£Ä³Š£»ī¶ÆŠ”×éĶ¬Ń§²ÉÓĆŅŌĻĀ×°ÖĆŗĶ²½ÖčÄ£Äā¹¤ŅµÉĻµŖŃõ»ÆĪļµÄ“¦Ąķ¹ż³Ģ”£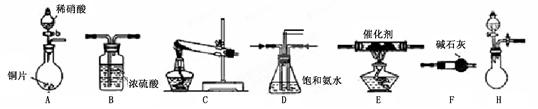
I£®Ģ½¾æÖĘČ”NH3µÄ·½·Ø
£Ø1£©ŌŚÉĻŹö×°ÖĆÖŠ£¬HÄÜæģĖŁ”¢¼ņ±ćÖĘČ”NH3£¬×°ÖĆÖŠŠčŅŖĢķ¼ÓµÄ·“Ó¦ŹŌ¼ĮĪŖ ”£
£Ø2£©ĪŖĢ½¾æøüŗƵďµŃ銧¹ū£¬»ī¶ÆŠ”×éĶ¬Ń§²ÉÓĆÉĻŹöC×°ÖĆĄ“ÖĘČ”°±Ęų£¬ŌŚæŲÖĘŹµŃéĢõ¼žĻąĶ¬µÄĒéæöĻĀ£¬»ńµĆĻĀ±ķÖŠŹµŃ鏿¾Ż”£
| ŹŌ¼Į×éŗĻŠņŗÅ | ¹ĢĢåŹŌ¼Į | NH3Ģå»ż£ØmL£© | |
| a | 6.0 g Ca(OH)2£Ø¹żĮ棩 | 5.4 g NH4Cl | 1344 |
| b | 5.4g (NH4)2SO4 | 1364 | |
| c | 6.0 g NaOH£Ø¹żĮ棩 | 5.4 g NH4Cl | 1568 |
| d | 5.4g (NH4)2SO4 | 1559 | |
| e | 6.0 g CaO£Ø¹żĮ棩 | 5.4 g NH4Cl | 1753 |
| f | 5.4 g (NH4)2SO4 | 1792 | |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com