
��ҵ�����������ᣨ�е㣺90oC��ʱ��ͬʱ�������������ƣ��乤���������£�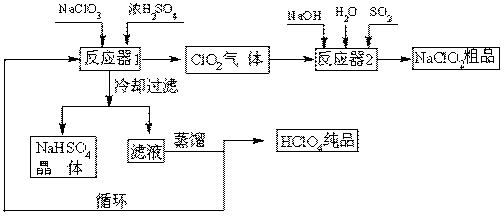
��1����ȴ���˵�Ŀ�� ��
��2��ͨ�뷴Ӧ��2��SO2������ ����Ӧ��2�з�����Ӧ�����ӷ���ʽΪ ��
��3��ѭ��ʹ�õ������� ��
��4������ͨ��������Һ�ķ����õ��������ԭ������� ��
��5��ͨ�����NaClO3ˮ��Һ�ķ���Ҳ�����Ʊ�NaClO4�����������Ʊ�HClO4��д�������ĵ缫��Ӧʽ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
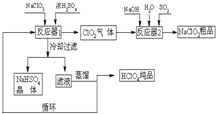 ��ҵ�����������ᣨ�е㣺90��C��ʱ��ͬʱ�������������ƣ��乤���������£�
��ҵ�����������ᣨ�е㣺90��C��ʱ��ͬʱ�������������ƣ��乤���������£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�����������ᣨ�е㣺90oC��ʱ��ͬʱ�������������ƣ��乤���������£�
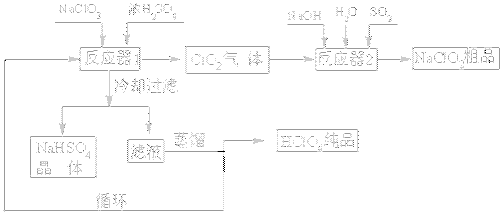
��1����ȴ���˵�Ŀ�� ��
��2��ͨ�뷴Ӧ��2��SO2������ ����Ӧ��2�з�����Ӧ�����ӷ���ʽΪ ��
��3��ѭ��ʹ�õ������� ��
��4������ͨ��������Һ�ķ����õ��������ԭ������� ��
��5��ͨ�����NaClO3ˮ��Һ�ķ���Ҳ�����Ʊ�NaClO4�����������Ʊ�HClO4��д�������ĵ缫��Ӧʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ������ѧ������ѧ�ڵڶ����¿���ѧ�Ծ� ���ͣ������
��12�֣���ҵ�����������ᣨ�е㣺90oC��ʱ��ͬʱ�������������ƣ��乤���������£�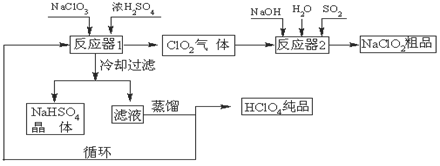
��1����ȴ���˵�Ŀ���ǽ���NaHSO4�� �������NaHSO4���塣
��2����Ӧ��2�з�����Ӧ�����ӷ���ʽΪ ��SO2���������� ����
��3��ѭ��ʹ�õ������� ��
��4������ͨ��������Һ�ķ����õ��������ԭ������� ��
��5����ҵ���ò���������ͭ�����������������Ҳ���Ƶø����ᣬ���������ɵõ�20%�ĸ����ᡣд�������ĵ缫��Ӧʽ������������������Ի�ѧʽ���֣� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��㶫ʡ�ع��и������в������ۻ�ѧ�Ծ��������棩 ���ͣ������
��ҵ�����������ᣨ�е㣺90��C��ʱ��ͬʱ�������������ƣ��乤���������£�
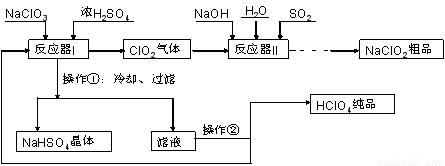
��1��ʵ���ҽ��й��˲����ij��ò���������???????????? ��
��2����Ӧ��I�е��¶����Ϊ???????? ������ţ���������������Ϊ????????? ��
A. 0��C ��????????? B. 20��C ��??????? C. 80��C ��????????? D. 120��C??
��3����Ӧ��II�з�����Ӧ�����ӷ���ʽΪ?????????????? ��
��4���ӿ췴Ӧ��II�з�Ӧ���ʵĴ�ʩ��?????????????? ��д��һ�ִ�ʩ���ɣ��ȡ��ӷ�Ӧ��II�л��NaClO2?? ��Ʒ��ʵ�����������???????? ������ţ���ͬ������һ���ᴿ�IJ�������Ϊ???????? ��
A������? B���ؽᾧ? C������? D������Ũ��? E����������? F����ȴ�ᾧ? G����ȡ��Һ
��5�����������п�ѭ��ʹ�õ�����Ϊ???????? ������Ʒ��NaClO2��NaHSO4���???????? ���ѧʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��㶫ʡ��У������ѧ����ĩ���������ۣ���ѧ���� ���ͣ������
��ҵ�����������ᣨ�е㣺90oC��ʱ��ͬʱ�������������ƣ��乤���������£�
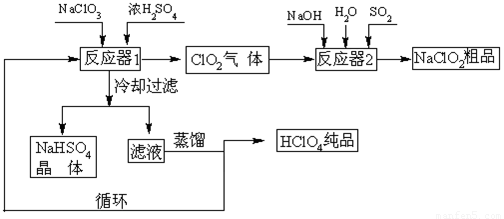
��1����ȴ���˵�Ŀ�� ��
��2��ͨ�뷴Ӧ��2��SO2������ ����Ӧ��2�з�����Ӧ�����ӷ���ʽΪ ��
��3��ѭ��ʹ�õ������� ��
��4������ͨ��������Һ�ķ����õ��������ԭ������� ��
��5��ͨ�����NaClO3ˮ��Һ�ķ���Ҳ�����Ʊ�NaClO4�����������Ʊ�HClO4��д�������ĵ缫��Ӧʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com