
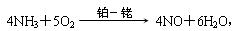
»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©ŗĻ³É°±µÄŌĮĻĶس£ø÷Ą“×ŌŗĪ“¦£æ_______________________”£
£Ø2£©ŅŃÖŖN2£Øg£©£«3H2£Øg£©![]() 2NH3£Øg£©£¬¦¤H£½£92 kJ”£Ēė½āŹĶ£ŗ
2NH3£Øg£©£¬¦¤H£½£92 kJ”£Ēė½āŹĶ£ŗ
ĪŖÓŠŠ§Ģįøß°±µÄ²śĀŹ£¬Źµ¼ŹÉś²śÖŠŅĖ²ÉČ”ÄÄŠ©“ėŹ©£æ_______________________”£
£Ø3£©Š“³ö°±“ß»ÆŃõ»ÆµÄ»Æѧ·½³ĢŹ½”£²¬īīŗĻ½šĶųÓŠŗĪ×÷ÓĆ£æĪŖŹ²Ć“²¬īīŗĻ½šĶųĪ“Ō¤ČČŅ²»į·¢ČČ£æ
£Ø4£©Éś²śĻõĖįµÄ¹ż³ĢÖŠ³£»į²śÉśŅ»Š©µŖµÄŃõ»ÆĪļ£¬ČēŗĪĻū³żĖüĆĒ¶Ō“óĘųµÄĪŪČ¾£æŠ“³ö·“Ó¦µÄ»Æѧ·½³ĢŹ½”£
£Ø5£©¾Ł³öĻõĖįļ§µÄĮ½ÖÖÖ÷ŅŖÓĆĶ¾”£Ēė½āŹĶĪŖŹ²Ć“øĆÓĆĶ¾¶ŌĻÖ“śÉē»į·Ē³£ÖŲŅŖ”£
£Ø6£©ŌŚŅ»¶ØĪĀ¶ČŗĶŃ¹ĒæµÄĆܱÕČŻĘ÷ÖŠ£¬½«Ę½¾łĻą¶Ō·Ö×ÓÖŹĮæĪŖ8.5µÄH2ŗĶN2»ģŗĻ£¬µ±øĆ·“Ó¦“ļµ½Ę½ŗāŹ±£¬²ā³öĘ½ŗā»ģŗĻĘųµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæĪŖ10£¬“ĖŹ±N2µÄ×Ŗ»ÆĀŹĪŖ________£¬Ę½ŗā»ģŗĻĘųĢåÖŠNH3µÄĢå»ż·ÖŹżĪŖ________”£
£Ø7£©ĒėÄćĪŖĻõĖį³§µÄєַĢį³öŗĻĄķ»Æ½ØŅ锣
£Ø1£©N2Č”×ŌÓŚæÕĘų£¬H2æÉĄ“×ŌÓŚĖ®ĆŗĘų
£Ø2£©æɲÉČ”ŌöŃ¹”¢ŹŹµ±ĪĀ¶ČŗĶŹ¹ÓĆĢś“„Ć½µČ“ėŹ©
£Ø3£©![]() ²¬īīŗĻ½šĶųĘš“ß»Æ×÷ÓĆ”£ŅņĪŖ°±µÄ“ß»ÆŃõ»Æ·“Ó¦ŹĒ·ÅČČ·“Ó¦£¬æɱ£Ö¤²¬īīŗĻ½šĶų“ļµ½Ņ»¶ØĪĀ¶Č²»±ŲŌ¤ČČ”£
²¬īīŗĻ½šĶųĘš“ß»Æ×÷ÓĆ”£ŅņĪŖ°±µÄ“ß»ÆŃõ»Æ·“Ó¦ŹĒ·ÅČČ·“Ó¦£¬æɱ£Ö¤²¬īīŗĻ½šĶų“ļµ½Ņ»¶ØĪĀ¶Č²»±ŲŌ¤ČČ”£
£Ø4£©æÉÓĆ¼īŅŗĪüŹÕµŖµÄŃõ»ÆĪļ”£ NO£«NO2£«2NaOH====2NaNO2£«H2O
£Ø5£©NH4NO3æÉÓĆ×÷»Æ·ŹŗĶÕØŅ©”£Ē°ÕßæÉ“ŁŹ¹Ö²ĪļÉś³¤£¬Ōö¼ÓÅ©×÷Īļ²śĮ棻ŗóÕßæÉÓĆÓŚ¾üŹĀ”¢½ØÉčÉĻÖĘŌģÕØŅ©£¬¹ŹøĆĪļÖŹ¶ŌĻÖ“śÉē»įµÄ·¢Õ¹ÓėŗĶĘ½µÄ×÷ÓĆÖŲ“ó”£
£Ø6£©30% 17.6%
£Ø7£©³§Ö·µÄŃ”ŌńÓ¦¾ß±øŅŌĻĀÓŠĄūĢõ¼ž£ŗŌĮĻŗĶ²śĘ·ŌĖŹä·½±ć”¢Éś²ś¶ÆĮ¦Ą“Ō“³ä×ć”¢·ĻĪļŅ×ÓŚ“¦Ąķ”¢µŲ¼Ū½Ļ±ćŅĖµČ”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com