
�����̿�MnCO3��������Fe2O3��FeO��HgCO3��2HgO�����ʣ���ҵ�������̿���ȡ�̣������������£�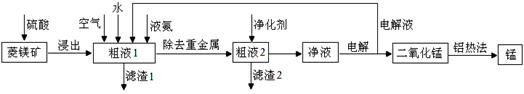
��ش��������⣺
��1�����Һ1�м����ˮ�����Ҫ �������ܴﵽ����Ҫ��
��2���������õĿ�������Ĥ���뷨�Ʊ��ĸ����������÷�����ԭ���� ��
��3����������Ҫ�ɷ�Ϊ(NH4)2S����Һ2�з�����Ҫ��Ӧ�����ӷ���ʽΪ ��
��4��д�������ĵ缫��Ӧʽ ��˵�����Һѭ����ԭ�� ��
��5��д�����ȷ����̵Ļ�ѧ����ʽ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��109g5.51����NaOH��Һ��������CuSO4��Һ��200g10.00����K2SO4��Һ���缫��Ϊʯī�缫��
��ͨ��Դ������һ��ʱ���ñ���K2SO4Ũ��Ϊ10.47��������c�缫�������ӡ��ݴ˻ش����⣺
��1���缫b�Ϸ����ĵ缫��ӦΪ___________________________________��
��2���缫b�����ɵ������ڱ�״���µ����Ϊ__________________����ʱ���ձ���NaOH��Һ�����ʵ���Ũ��Ϊ������Һ���ܶ�Ϊ1g/cm3��_______________��
��3���缫c�������仯��___________g����ʹ�������еĵ��Һ�ָ�����ʼ״̬��Ӧ������Һ�м���������___________������ĸ��ţ���
| A��Cu(OH)2 | B��Cu2O | C��CuCO3 | D��Cu2(OH)2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�á���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ������
��ش��������⣺
��1����X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ����
�ٵ�����X���ϵĵ缫��ӦʽΪ ��
��X�������۲쵽�������� ����
��Y�缫�ϵĵ缫��ӦʽΪ ��
����õ缫��Ӧ����ķ����� ��
��2����Ҫ�õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ����
��X�缫�IJ����� ���缫��Ӧʽ�� ��
��Y�缫�IJ����� ���缫��Ӧʽ�� ��
��˵�������ʷ����ĵ缫��Ӧ����д����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
(14��)2013������������������Ű������ȵ��������У�ȼú������β����
��ɿ�����Ⱦ��ԭ��֮һ��
(l)����β����������Ҫԭ��Ϊ��2NO(g) + 2CO(g) 2CO2(g)+ N2(g)��H <0
2CO2(g)+ N2(g)��H <0
�ٸ÷�Ӧƽ�ⳣ������ʽ____________________________
�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬����________________������ţ���
��2��ֱ���ŷ�úȼ�ղ������������������صĻ������⡣úȼ�ղ����������������������CH4����ԭNOx�������������������Ⱦ��
��֪��CH4(g)+2NO2(g)��N2(g)��CO2(g)+2H2O(g)����H����867 kJ/mol ��
2NO2(g)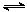 N2O4(g) ��H����56.9 kJ/mol ��
N2O4(g) ��H����56.9 kJ/mol ��
H2O(g)��H2O(l) ��H����44.0 kJ/mol ��
д��CH4����ԭN2O4(g)����N2��H2O��1�����Ȼ�ѧ����ʽ��_____________________��
��3������ȼ�ϵ�ؿ����������������ʡ���ͼ�����ü���ȼ�ϵ�ص��100mLlmol/Lʳ��ˮ�����һ��ʱ����ռ�����״���µ�����2.24L���������Һ������䣩��
�ټ���ȼ�ϵ�صĸ�����Ӧʽ��______________________________________.
�ڵ�����Һ��pH=____����������������������Һ��Ӧ��
�������������������ڱ�״������________L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
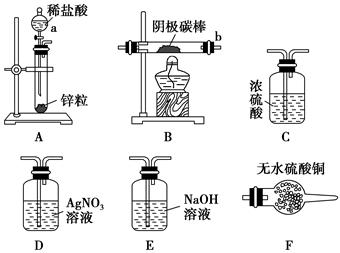
��8�֣��������֪ʶ�ش��������⣺
��1���ô�����̼�����ϡ���ᷴӦ��ȡ������̼���壬ʵ����̲�õ�CO2���������ʱ��仯����ͼ��ʾ��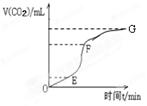
��________�λ�ѧ��Ӧ������죬_______���ռ��Ķ�����̼������ࡣ
�ڳ���������ˮ�⣬���������������е� ������ţ�ʱ�����ܹ�����������Ӧ�����ʡ�
A����������Һ B��̼��Ʒ�ĩ C��ϡ���� D��Ũ����
��2��ԭ�����һ������װ�á�
�������������Ͽ��������ԭ��صķ�Ӧ�� ������ţ���
A��NaOH +HCl==NaCl+H2O B��2FeCl3��Cu=2FeCl2��CuCl2
C��CuSO4 +2NaOH== Cu(OH)2+NaSO4 D��C2H6O +3O2==3H2O+2CO2
��ʵ��������пƬ�����ᷴӦ��ȡ����ʱ����Ӧ��Һ�еμӼ���CuSO4��Һ�����Է��ֲ��������������Լӿ죬��ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijС��ͬѧ��̼��Ϊ�缫���CuCl2��Һʱ����������̼���ϳ����к�ɫ���������⣬����������ɫ����������Ϊ̽������̼���ϵIJ��ͬѧ���Ķ����ϲ���������¹��̣�
��.�й����ϣ�ͭ�Ļ�������ɫ��������
| ���� | ��ɫ������ | ���� | ��ɫ������ |
| ������ͭCu(OH)2 | ��ɫ���岻����ˮ | ����ͭ(CuSO4) | ��Һ����ɫ |
| ������ͭ(Cu2O) | ��ɫ���岻����ˮ | �Ȼ�ͭ(CuCl2) | Ũ��Һ����ɫ��ϡ��Һ����ɫ |
| �Ȼ���ͭ(CuCl) | ��ɫ���岻����ˮ | ��ʽ�Ȼ�ͭ | ��ɫ���岻����ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(16��)ij�о�С�����ô�ʳ��(��Ca2+��Mg2+��SO ��)���ֹ�(��C������Cl2��Ӧ�Ĺ�������)��ȡ���裬������µĹ������̣�
��)���ֹ�(��C������Cl2��Ӧ�Ĺ�������)��ȡ���裬������µĹ������̣�
�Իش��������⣺
��1����ҵ��һ�������ù�����̿�ڸ����»�ԭʯӢɰ����ȡ�ֹ裬д���ù��̵Ļ�ѧ����ʽ��_______________________________________________________________________��
��2�����ƴ���ˮ�����Լ�Ϊ��BaC12����Na2CO3����HC1����NaOH����μӵ��Ⱥ�˳�������е�________(�����и�������)��
a���٢ڢܢ� b���ܢڢ٢� c���ܢ٢ۢ� d���ڢܢ٢�
��֪�� ������ô���ˮ��
������ô���ˮ�� ��Ũ�Ⱦ�Ϊ0.01 mol��L-1������1 L�ô���ˮ������һ����Na2CO3��Һ�����ȳ��ֵij�����__________��
��Ũ�Ⱦ�Ϊ0.01 mol��L-1������1 L�ô���ˮ������һ����Na2CO3��Һ�����ȳ��ֵij�����__________��
��3����֪SiCl4�ķе���57.6�棬CC14�ķе���76.8�档�ڷ�Ӧ��I�еõ���SiCl4��Ʒ�к���CCl4�����еõ�����SiCl4�ɲ��õķ��������и����е�________(�����)��
a������ b������ c����Һ d������
��Ӧ�����з�����Ӧ�Ļ�ѧ����ʽΪ__________________________________________��
��4����ͼ�������ӽ���Ĥ����ⱥ��ʳ��ˮ��ʾ��ͼ������������������������_____����ƷA�Ļ�ѧʽΪ____________��
��������Ĥ���۵�ⱥ��ʳ��ˮ����ȡ�������ƣ���д���÷�Ӧ�Ļ�ѧ����ʽ__ ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪̼̼�������Ƽ���������ת,ij���Ľṹ��ʽ����ͼ��ʾ,����˵������ȷ����
| A������������ԭ�Ӿ��ɹ��� |
| B��������������10��̼ԭ�Ӵ���ͬһƽ���� |
| C��������������11��̼ԭ�Ӵ���ͬһƽ���� |
| D�������뱽��Ϊͬϵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ȩ������������ɵĻ�����У�����̼����������ΪA%����˻�����к������������Ϊ
| A��6A% | B��A/6% | C��10A% | D��(100��7A/6)% |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com