
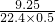 =0.826£¨g/l£©£»
=0.826£¨g/l£©£»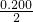 mol=0.900mol£¬¸ù¾ف»ى؛دئّجهµؤأـ¶ب=
mol=0.900mol£¬¸ù¾ف»ى؛دئّجهµؤأـ¶ب= =
=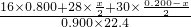 =0.780£¬
=0.780£¬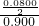 ،ء100%=4.44%£»
،ء100%=4.44%£»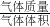 ہ´¼ئثم£»
ہ´¼ئثم£»

 شؤ¶ء؟ى³µدµءذ´ً°¸
شؤ¶ء؟ى³µدµءذ´ً°¸
| ؤ꼶 | ¸كضذ؟خ³ج | ؤ꼶 | ³ُضذ؟خ³ج |
| ¸كز» | ¸كز»أâ·ر؟خ³جحئ¼ِ£، | ³ُز» | ³ُز»أâ·ر؟خ³جحئ¼ِ£، |
| ¸ك¶ | ¸ك¶أâ·ر؟خ³جحئ¼ِ£، | ³ُ¶ | ³ُ¶أâ·ر؟خ³جحئ¼ِ£، |
| ¸كب | ¸كبأâ·ر؟خ³جحئ¼ِ£، | ³ُب | ³ُبأâ·ر؟خ³جحئ¼ِ£، |
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
| 1 | 2 |
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛شؤ¶ءہي½â


| ||
| ||
 ¢ع°±ئّ؟ةت¹تھبَµؤ؛ىة«µؤت¯بïتشض½±نہ¶µؤشزٍ£¨سأ·½³جت½±يت¾£©
¢ع°±ئّ؟ةت¹تھبَµؤ؛ىة«µؤت¯بïتشض½±نہ¶µؤشزٍ£¨سأ·½³جت½±يت¾£©
| ||
| ،÷ |
| ||
| ،÷ |
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛خïہي½جرذتز جâذح£؛038
£¨2£©CH4شعز»¶¨جُ¼دآ´ك»¯رُ»¯؟ةزشةْ³ةC2H4،¢C2H6£¨ث®؛حئنثû·´س¦²ْخï؛ِآش²»¼ئ£©،£ب،ز»¶¨ء؟CH4¾´ك»¯رُ»¯؛َµأµ½ز»ضض»ى؛حئّجه£¬ثüشع±ê×¼×´؟ِدآµؤأـ¶بخھ0.780g/L،£زرضھ·´س¦ضذCH4دû؛ؤءث20.0%£¬¼ئثم»ى؛حئّجهضذC2H4µؤجه»·ضت،££¨±¾جâ¼ئثم¹³جضذاë±£ءô3خ»سذذ§ت×ض£©
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛038
£¨1£©زرضھؤ³»ى؛حئّجهµؤجه»°ظ·ض×é³ةخھ80.0%CH4،¢15.0%C2H4؛ح5.00%C2H6،£اë¼ئثم0.500 mol¸أ»ى؛حئّجهµؤضتء؟؛ح±ê×¼×´؟ِدآµؤأـ¶ب(g/L)،£
£¨2£©CH4شعز»¶¨جُ¼دآ´ك»¯رُ»¯؟ةزشةْ³ةC2H4،¢C2H6£¨ث®؛حئنثû·´س¦²ْخï؛ِآش²»¼ئ£©،£ب،ز»¶¨ء؟CH4¾´ك»¯رُ»¯؛َµأµ½ز»ضض»ى؛حئّجه£¬ثüشع±ê×¼×´؟ِدآµؤأـ¶بخھ0.780g/L،£زرضھ·´س¦ضذCH4دû؛ؤءث20.0%£¬¼ئثم»ى؛حئّجهضذC2H4µؤجه»·ضت،££¨±¾جâ¼ئثم¹³جضذاë±£ءô3خ»سذذ§ت×ض£©
²é؟´´ً°¸؛ح½âخِ>>
°ظ¶بضآذإ - ء·د°²لءذ±ي - تشجâءذ±ي
؛±±ت،»¥ءھحّخ¥·¨؛ح²»ء¼ذإد¢¾ظ±¨ئ½ج¨ | حّةدسذ؛¦ذإد¢¾ظ±¨×¨اّ | µçذإص©ئ¾ظ±¨×¨اّ | ةوہْت·ذéخقض÷زهسذ؛¦ذإد¢¾ظ±¨×¨اّ | ةوئَاضب¨¾ظ±¨×¨اّ
خ¥·¨؛ح²»ء¼ذإد¢¾ظ±¨µç»°£؛027-86699610 ¾ظ±¨ستدن£؛58377363@163.com