
ЁОЬтФПЁПМзДМЪЧживЊЕФЛЏЙЄдСЯЃЌгжПЩГЦЮЊШМСЯЁЃРћгУКЯГЩЦјЃЈжївЊГЩЗжЮЊCOЁЂCO2КЭH2ЃЉдкДпЛЏМСЕФзїгУЯТКЯГЩМзДМЃЌЗЂЩњЕФжїЗДгІШчЯТЃК
ЂйCO(g)+2H2(g)![]() CH3OH(g) ЁїH1
CH3OH(g) ЁїH1
ЂкCO2(g)+3H2(g)![]() CH3OHЃЈgЃЉ+H2O(g) ЁїH2
CH3OHЃЈgЃЉ+H2O(g) ЁїH2
ЂлCO2(g)+H2(g)![]() CO(g)+H2O(g) ЁїH3
CO(g)+H2O(g) ЁїH3
ЃЈ1ЃЉвбжЊЗДгІЂйжаЕФЯрЙиЕФЛЏбЇМќМќФмЪ§ОнШчЯТЃК
ЛЏбЇМќ | HЁЊH | CЁЊO | CЁдO | HЁЊO | CЁЊH |
E/(kJЁЄmol-1) | 436 | 343 | 1076 | 465 | 413 |
гЩДЫМЦЫуЁїH1=__________kJЁЄmol-1ЃЌвбжЊЁїH2=-58kJЁЄmol-1ЃЌдђЁїH3=_________kJЁЄmol-1
ЃЈ2ЃЉЗДгІЂйЕФЛЏбЇЦНКтГЃЪ§KЕФБэДяЪНЮЊ_______________ЃЛЂлЕФЛЏбЇЦНКтГЃЪ§KЕФБэДяЪНЮЊ_____________ЃЛ
ЁОД№АИЁП-99 +41 K=![]() K=
K=![]()
ЁОНтЮіЁП
(1)ЗДгІШШ=ЗДгІЮязмМќФм-ЩњГЩЮязмМќФмЃЛИљОнИЧЫЙЖЈТЩЃКЗДгІЂк-ЗДгІЂй=ЗДгІЂлЃЌЗДгІШШвВНјааЯргІЕФМЦЫуЃЛ(2)ЛЏбЇЦНКтГЃЪ§жИПЩФцЗДгІЕУЕНЦНКтЪБЃЌИїЩњГЩЮяХЈЖШЕФЛЏбЇМЦСПЪ§ДЮУнЕФГЫЛ§Г§вдИїЗДгІЮяХЈЖШЕФЛЏбЇМЦСПЪ§ДЮУнЕФГЫЛ§ЫљЕУЕФБШжЕЃЛОнДЫЗжЮіНтД№ЁЃ
(1)ЗДгІШШ=ЗДгІЮязмМќФм-ЩњГЩЮязмМќФмЃЌЙЪЁїH1=1076kJЁЄmol-1+2ЁС436kJЁЄmol-1-(3ЁС413+343+465)kJЁЄmol-1=-99kJЁЄmol-1ЃЌИљОнИЧЫЙЖЈТЩЃКЗДгІЂк-ЗДгІЂй=ЗДгІЂлЃЌЙЪЁїH3=ЁїH2-ЁїH1=-58kJЁЄmol-1-(-99kJЁЄmol-1)=+41kJЁЄmol-1ЃЌЙЪД№АИЮЊЃК-99ЃЛ+41ЃЛ
(2)ЗДгІЂйCO(g)+2H2(g)CH3OH(g)ЕФЦНКтГЃЪ§БэДяЪНK=![]() ЃЌЗДгІЂлCO2(g)+H2(g)
ЃЌЗДгІЂлCO2(g)+H2(g)![]() CO(g)+H2O(g)ЕФЛЏбЇЦНКтГЃЪ§K=
CO(g)+H2O(g)ЕФЛЏбЇЦНКтГЃЪ§K=![]() ЃЌЙЪД№АИЮЊЃК K=
ЃЌЙЪД№АИЮЊЃК K=![]() ЃЛK=
ЃЛK=![]() ЁЃ
ЁЃ


 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПеђЭДвЉЮя![]() ЕФКЯГЩЗНЗЈШчЯТЃК
ЕФКЯГЩЗНЗЈШчЯТЃК

вбжЊЃК
(1)![]() ЕФУћГЦЮЊ_________________ЃЛЂкЕФЗДгІРраЭЮЊ_________________ЗДгІЁЃ
ЕФУћГЦЮЊ_________________ЃЛЂкЕФЗДгІРраЭЮЊ_________________ЗДгІЁЃ
(2)![]() ЕФНсЙЙМђЪНЮЊ_________________.
ЕФНсЙЙМђЪНЮЊ_________________.![]() жаКЌбѕЙйФмЭХЕФУћГЦЮЊ_________________.
жаКЌбѕЙйФмЭХЕФУћГЦЮЊ_________________.
(3)ЂлЕФЛЏбЇЗНГЬЪНЮЊ__________________________________ЁЃ
(4)гаЛњЮя![]() ЗжзгзщГЩБШ
ЗжзгзщГЩБШ![]() ЩйСНИіЧтдзгЃЌЗћКЯЯТСавЊЧѓЕФ
ЩйСНИіЧтдзгЃЌЗћКЯЯТСавЊЧѓЕФ![]() ЕФЭЌЗжвьЙЙЬхга_________жжЁЃ
ЕФЭЌЗжвьЙЙЬхга_________жжЁЃ
A гі![]() ЯдзЯЩЋ B БНЛЗЩЯгаСНИіШЁДњЛљ
ЯдзЯЩЋ B БНЛЗЩЯгаСНИіШЁДњЛљ
(5)вбжЊЂмгавЛЖЈЕФЗДгІЯоЖШЃЌЗДгІНјааЪБМгШыпСрЄ(![]() ЃЌЪєгкгаЛњМю)ФмЬсИп
ЃЌЪєгкгаЛњМю)ФмЬсИп![]() ЕФВњТЪЃЌдвђЪЧ___________________________________________________ЁЃ
ЕФВњТЪЃЌдвђЪЧ___________________________________________________ЁЃ
(6) ЃЌЪЧвЛжжживЊЕФЛЏЙЄжаМфЬхЁЃвдЛЗМКДМ(
ЃЌЪЧвЛжжживЊЕФЛЏЙЄжаМфЬхЁЃвдЛЗМКДМ(![]() )КЭввДМЮЊЦ№ЪМдСЯЃЌНсКЯМКжЊаХЯЂбЁдёБивЊЕФЮоЛњЪдМСЃЌаДГі
)КЭввДМЮЊЦ№ЪМдСЯЃЌНсКЯМКжЊаХЯЂбЁдёБивЊЕФЮоЛњЪдМСЃЌаДГі ЕФКЯГЩТЗЯпЁЃ
ЕФКЯГЩТЗЯпЁЃ
(вбжЊЃК![]()
![]() ЁЂ
ЁЂ![]() ЮЊЬўЛљЁЃгУНсЙЙМђЪНБэЪОгаЛњЮяЃЌгУМ§ЭЗБэЪОзЊЛЏЙиЯЕЃЌМ§ЭЗЩЯзЂУїЪдМСКЭЗДгІЬѕМў)________________________________
ЮЊЬўЛљЁЃгУНсЙЙМђЪНБэЪОгаЛњЮяЃЌгУМ§ЭЗБэЪОзЊЛЏЙиЯЕЃЌМ§ЭЗЩЯзЂУїЪдМСКЭЗДгІЬѕМў)________________________________
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПгаЫФжжЖЬжмЦкдЊЫиЃЎЫќУЧЕФНсЙЙЁЂаджЪЕШаХЯЂШчЯТБэЫљЪіЃК

ЧыИљОнБэжааХЯЂЬюаДЃК
ЃЈ1ЃЉAдзгЕФКЫЭтЕчзгХХВМЪНЮЊ________________ЁЃ
ЃЈ2ЃЉBдЊЫидкжмЦкБэжаЕФЮЛжУЪЧ____ЃЛРызгАыОЖЃКB_____A(ЬюЁАДѓгкЁБЛђЁАаЁгкЁБ)ЁЃ
ЃЈ3ЃЉCдзгЕФЕчзгХХВМЭМЪЧ________ЃЌЦфдзгКЫЭтга________ИіЮДГЩЖдЕчзгЃЌФмСПзюИпЕФЕчзгЮЊ________ЙьЕРЩЯЕФЕчзгЃЌЦфЙьЕРГЪ________аЮЁЃ
ЃЈ4ЃЉDдзгЕФЭтЮЇЕчзгХХВМЪНЮЊ____________ЃЌDЃЕФНсЙЙЪОвтЭМЪЧ____________ЁЃ
ЃЈ5ЃЉBЕФзюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮягыAЕФзюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮяЗДгІЕФЛЏбЇЗНГЬЪНЮЊ________ЃЛBЕФзюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮягыDЕФЧтЛЏЮяЕФЫЎШмвКЗДгІЕФЛЏбЇЗНГЬЪНЮЊ__________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁППЩФцЗДгІaA(s)+bB(g)![]() cC(g) +dD(g)ЃЌ ЕБЦфЫћЬѕМўВЛБфЪБЃЌФГЮяжЪдкЛьКЯЮяжаЕФКЌСПгыЮТЖШ(T)ЁЂбЙЧП(p)ЕФЙиЯЕШчЭМЫљЪОЃЌвдЯТе§ШЗЕФЪЧЃЈ ЃЉ
cC(g) +dD(g)ЃЌ ЕБЦфЫћЬѕМўВЛБфЪБЃЌФГЮяжЪдкЛьКЯЮяжаЕФКЌСПгыЮТЖШ(T)ЁЂбЙЧП(p)ЕФЙиЯЕШчЭМЫљЪОЃЌвдЯТе§ШЗЕФЪЧЃЈ ЃЉ
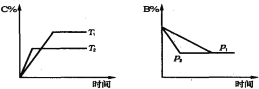
A. T1>T2ЃЌе§ЗДгІЗХШШ
B. Tl<T2ЃЌе§ЗДгІЮќШШ
C. P1>P2ЃЌa+b>c+d
D. PlЃМP2ЃЌb=c+d
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаШШЛЏбЇЗНГЬЪНЪщаДе§ШЗЕФЪЧ(ІЄHЕФОјЖджЕОље§ШЗ)
A. C2H5OH(l)+3O2(g)![]() 2CO2(g)+3H2O(g)ЁЁІЄH=1 367.0 kJ/mol(ШМЩеШШ)
2CO2(g)+3H2O(g)ЁЁІЄH=1 367.0 kJ/mol(ШМЩеШШ)
B. NaOH(aq)+HCl(aq)![]() NaCl(aq)+H2O(l)ЁЁІЄH=+57.3 kJ/mol(жаКЭШШ)
NaCl(aq)+H2O(l)ЁЁІЄH=+57.3 kJ/mol(жаКЭШШ)
C. S(s)+O2(g)![]() SO2(g)ЁЁІЄH=296.8 kJ/mol(ЗДгІШШ)
SO2(g)ЁЁІЄH=296.8 kJ/mol(ЗДгІШШ)
D. 2NO2![]() O2+2NOЁЁІЄH=+116.2 kJ/mol(ЗДгІШШ)
O2+2NOЁЁІЄH=+116.2 kJ/mol(ЗДгІШШ)
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПАБЮЊживЊЕФЛЏЙЄдСЯЃЌгаЙуЗКгУЭОЁЃ
(1)КЯГЩАБжаЕФЧтЦјПЩгЩЯТСаЗДгІжЦШЁЃК
aЃЎCH4(g)ЃЋH2O(g)![]() CO(g)ЃЋ3H2(g) ІЄH1ЃНЃЋ216.4 kJЁЄmolЃ1
CO(g)ЃЋ3H2(g) ІЄH1ЃНЃЋ216.4 kJЁЄmolЃ1
bЃЎCO(g)ЃЋH2O(g)![]() CO2(g)ЃЋH2(g) ІЄH2ЃНЃ41.2 kJЁЄmolЃ1
CO2(g)ЃЋH2(g) ІЄH2ЃНЃ41.2 kJЁЄmolЃ1
дђЗДгІCH4(g)ЃЋ2H2O(g)![]() CO2(g)ЃЋ4H2(g)ЁЁІЄHЃН_________________________ЁЃ
CO2(g)ЃЋ4H2(g)ЁЁІЄHЃН_________________________ЁЃ
(2)Ц№ЪМЪБЭЖШыЕЊЦјКЭЧтЦјЕФЮяжЪЕФСПЗжБ№ЮЊ1 molЁЂ3 molЃЌдкВЛЭЌЮТЖШКЭбЙЧПЯТКЯГЩАБЁЃЦНКтЪБЛьКЯЮяжаАБЕФЬхЛ§ЗжЪ§гыЮТЖШЕФЙиЯЕШчЭМЁЃ

ЂйКубЙЪБЃЌЗДгІвЛЖЈДяЕНЦНКтзДЬЌЕФБъжОЪЧ________(ЬюађКХ)ЁЃ
A N2КЭH2ЕФзЊЛЏТЪЯрЕШ B ЗДгІЬхЯЕУмЖШБЃГжВЛБф
C c(H2)/c(NH3 )БЃГжВЛБф D c(NH3 )/c(N2)ЃН2
Ђкp1________(ЬюЁА>ЁБЁЂЁА<ЁБЁЂЁАЃНЁБЛђЁАВЛШЗЖЈЁБЃЌЯТЭЌ)p2ЃЛЗДгІЕФЦНКтГЃЪ§ЃКBЕу________DЕуЁЃ
ЂлCЕуH2ЕФзЊЛЏТЪЮЊ________ЃЛдкAЁЂBСНЕуЬѕМўЯТЃЌИУЗДгІДгПЊЪМЕНЦНКтЪБЩњГЩАБЦјЕФЦНОљЫйТЪЃКv(A)________v(B)ЁЃ
(3)N2H4ПЩзїЛ№М§ЭЦНјМСЃЌNH3КЭNaClOдквЛЖЈЬѕМўЯТЗДгІПЩЩњГЩN2H4ЁЃ
ЂйаДГіNH3КЭNaClOЗДгІЩњГЩN2H4ЕФЛЏбЇЗНГЬЪН________________________________ЁЃ
ЂквбжЊ25 ЁцЪБЃЌN2H4ЕФЫЎШмвКГЪШѕМюадЃКN2H4ЃЋH2O![]() N2H5+ЃЋOHЃK1ЃН1ЁС10Ѓa N2H5+ЃЋH2O
N2H5+ЃЋOHЃK1ЃН1ЁС10Ѓa N2H5+ЃЋH2O![]() N2H62+ЃЋOHЃЁЁK2ЃН1ЁС10Ѓb25 ЁцЪБЃЌЯђN2H4ЫЎШмвКжаМгШыH2SO4ЃЌгћЪЙc(N2H5+)>c(N2H4)ЃЌЭЌЪБc(N2H5+)>c(N2H62+)ЃЌгІПижЦШмвКpHЕФЗЖЮЇЮЊ__________________________________(гУКЌaЁЂbЕФЪНзгБэЪО)ЁЃ
N2H62+ЃЋOHЃЁЁK2ЃН1ЁС10Ѓb25 ЁцЪБЃЌЯђN2H4ЫЎШмвКжаМгШыH2SO4ЃЌгћЪЙc(N2H5+)>c(N2H4)ЃЌЭЌЪБc(N2H5+)>c(N2H62+)ЃЌгІПижЦШмвКpHЕФЗЖЮЇЮЊ__________________________________(гУКЌaЁЂbЕФЪНзгБэЪО)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЪЕбщЪввдгаЛњЮяAЁЂEЮЊдСЯЃЌжЦБИОлѕЅЯЫЮЌMКЭвЉЮяжаМфЬхNЕФвЛжжКЯГЩТЗЯпШчЯТЃК

вбжЊЃКЂйAЕФКЫДХЙВеёЧтЦзга3зщЗх
Ђк
Ђл
ЧыЛиД№ЯТСаЮЪЬтЃК
(1)AЕФЛЏбЇУћГЦЮЊ____________ЁЃ
(2)JЕФНсЙЙМђЪНЮЊ_______________________ЁЃ
(3)BЁњCЁЂGЁњHЕФЗДгІРраЭЗжБ№ЮЊ______________ЁЂ______________ЁЃ
(4)ЙигкEЕФЫЕЗЈе§ШЗЕФЪЧ________(ЬюбЁЯюзжФИ)ЁЃ
A ЗжзгжаКЌгаЬМЬМЫЋМќ B ЫљгадзгОљдкЭЌвЛЦНУцЩЯ
C СкЖўТШДњЮягаСНжжНсЙЙ D ФмЪЙЫсадKMnO4ШмвКЭЪЩЋ
(5)DЃЋIЁњMЕФЛЏбЇЗНГЬЪНЮЊ_______________________________________________ЁЃ
(6)ЭЌЪБТњзуЯТСаЬѕМўЕФN(C8H12O3)ЕФЭЌЗжвьЙЙЬхга________жж(ВЛПМТЧСЂЬхвьЙЙ)ЁЃ
ЂйБЅКЭЮхдЊЬМЛЗЩЯСЌгаСНИіШЁДњЛљ ЂкФмгыNaHCO3ШмвКЗДгІ ЂлФмЗЂЩњвјОЕЗДгІ
(7)ВЮееЩЯЪіКЯГЩТЗЯпКЭаХЯЂЃЌвдЛЗИ§ДМКЭМзДМЮЊгаЛњдСЯ(ЮоЛњЪдМСШЮбЁ)ЃЌЩшМЦжЦБИ![]() ЕФКЯГЩТЗЯп_______________________________________________________ЁЃ
ЕФКЯГЩТЗЯп_______________________________________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПдквЛЖЈЬѕМўЯТЃЌШнЛ§ЮЊ2 LЕФУмБеШнЦїжаЃЌНЋ2 mol LЦјЬхКЭ3 mol MЦјЬхЛьКЯЃЌЗЂЩњШчЯТЗДгІЃК2L(g)ЃЋ3M(g)![]() xQ(g)ЃЋ3R(g)ЃЌ10sФЉЃЌЩњГЩ2.4 mol RЃЌВЂВтЕУQЕФХЈЖШЮЊ0.4 molЁЄLЃ1ЁЃМЦЫуЃК
xQ(g)ЃЋ3R(g)ЃЌ10sФЉЃЌЩњГЩ2.4 mol RЃЌВЂВтЕУQЕФХЈЖШЮЊ0.4 molЁЄLЃ1ЁЃМЦЫуЃК
ЃЈ1ЃЉ10 sФЉLЕФЮяжЪЕФСПХЈЖШЮЊ_____________ЁЃ
ЃЈ2ЃЉЧА10 sФкгУMБэЪОЕФЛЏбЇЗДгІЫйТЪЮЊ_____________ЁЃ
ЃЈ3ЃЉЛЏбЇЗНГЬЪНжаxжЕЮЊ_____________ЁЃ
ЃЈ4ЃЉдкКуЮТКуШнЬѕМўЃЌЭљШнЦїжаМгШы1 molКЄЦјЃЌЗДгІЫйТЪ________(діДѓЁЂМѕаЁЁЂВЛБф)ЁЃ
ЃЈ5ЃЉдкКуЮТКубЙЬѕМўЃЌЭљШнЦїжаМгШы1 molКЄЦјЃЌЗДгІЫйТЪ________(діДѓЁЂМѕаЁЁЂВЛБф)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПРћгУЕчЛЏбЇдРэПЩЭЌЪБНЋSO2ЁЂCO2БфЗЯЮЊБІЃЌзАжУШчЭМЫљЪО(ЕчМЋОљЮЊЖшадЕчМЋ)ЁЃЯТСаЫЕЗЈВЛе§ШЗЕФЪЧ

A. aЮЊИКМЋЃЌЗЂЩњбѕЛЏЗДгІ
B. зАжУЙЄзїЪБЃЌЕчзгДгcМЋСїШыbМЋ
C. ШєbМЋЯћКФ16gO2ЃЌдђYжазѓВрШмвКжЪСПМѕЧс16g
D. dЕчМЋЗДгІЪНЮЊCO2+6H++6eЃ=CH3OH+H2O
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com