
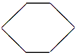
 £®
£®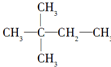 £¬ŌņAµÄ½į¹¹¼ņŹ½ĪŖ£ØCH3£©3CCH=CH2£®
£¬ŌņAµÄ½į¹¹¼ņŹ½ĪŖ£ØCH3£©3CCH=CH2£®·ÖĪö £Ø1£©Ģžŗ¬ÓŠC”¢HĮ½ÖÖŌŖĖŲ£¬Ä³ĢžA0.2molŌŚŃõĘųµÄ³ä·ÖČ¼ÉÕŗóÉś³É»ÆŗĻĪļB£¬Cø÷1.2mol£¬¼“Éś³ÉCO2”¢H2Oø÷1.2mol£¬Ōņ1molĢžÖŠŗ¬ÓŠ6molC£¬12molHŌ×Ó£¬A·Ö×ÓŹ½ĪŖC6H12£»
£Ø2£©øł¾ŻČ¼ÉÕ·½³ĢŹ½¼ĘĖćAµÄĪļÖŹµÄĮ攢ĻūŗÄŃõĘųµÄĪļÖŹµÄĮ棬ŌŁøł¾Żm=nM¼ĘĖćĢžAµÄÖŹĮ棬øł¾ŻV=nVm¼ĘĖćĻūŗÄŃõĘųµÄĢå»ż£»
£Ø3£©ČōĢžA²»ÄÜŹ¹äåĖ®ĶŹÉ«£¬ĖµĆ÷ÓŠ»śĪļÖŠ²»ŗ¬C=C¹ŁÄÜĶÅ£¬µ«ŌŚŅ»¶ØĢõ¼žĻĀ£¬ÄÜÓėĀČĘų·¢ÉśČ”“ś·“Ó¦£¬ĘäŅ»ĀČ“śĪļÖ»ÓŠŅ»ÖÖ£¬Ó¦ĪŖ»·¼ŗĶ飻
£Ø4£©ČōĢžAÄÜŹ¹äåĖ®ĶŹÉ«£¬ŌŚ“߻ƼĮ×÷ÓĆĻĀÓėH2¼Ó³ÉÉś³É £¬ĖµĆ÷·Ö×ÓÖŠŗ¬ÓŠ1øöC=C¼ü£¬ĻąĮŚĮ½øöĢ¼Ō×Ó¶¼ŗ¬ÓŠHŌ×ÓĪŖC=CĖ«¼üĪ»ÖĆ£¬¾Ż“ĖČ·¶ØAµÄ½į¹¹¼ņŹ½£»
£¬ĖµĆ÷·Ö×ÓÖŠŗ¬ÓŠ1øöC=C¼ü£¬ĻąĮŚĮ½øöĢ¼Ō×Ó¶¼ŗ¬ÓŠHŌ×ÓĪŖC=CĖ«¼üĪ»ÖĆ£¬¾Ż“ĖČ·¶ØAµÄ½į¹¹¼ņŹ½£»
£Ø5£©ČōĢžAÄÜŹ¹äåĖ®ĶŹÉ«£¬ĒŅ·Ö×ÓÖŠĖłÓŠĢ¼Ō×Ó¹²Ę½Ćę£¬ŌņC=CĖ«¼üÖŠ²»±„ŗĶCŌ×ÓĮ¬½ÓĖÄøö¼×»ł£¬¾Ż“ĖČ·¶ØAµÄ½į¹¹¼ņŹ½£»
£Ø6£©±ČAÉŁĮ½øöĢ¼Ō×ÓµÄAµÄĻ©ĢžĶ¬ĻµĪļ·Ö×ÓŹ½ĪŖC4H8£®
½ā“š ½ā£ŗ£Ø1£©Ģžŗ¬ÓŠC”¢HĮ½ÖÖŌŖĖŲ£¬Ä³ĢžA0.2molŌŚŃõĘųµÄ³ä·ÖČ¼ÉÕŗóÉś³É»ÆŗĻĪļB£¬Cø÷1.2mol£¬¼“Éś³ÉCO2”¢H2Oø÷1.2mol£¬Ōņ1molĢžÖŠŗ¬ÓŠ6molC£¬12molHŌ×Ó£¬·Ö×ÓŹ½ĪŖC6H12£¬
¹Ź“š°øĪŖ£ŗC6H12£»
£Ø2£©C6H12ĶźČ«Č¼ÉÕ£¬Éś³É3molCO2ŗĶH2O£¬Ōņ£ŗ
C6H12+9O2$\frac{\underline{\;µćČ¼\;}}{\;}$6CO2+6H2O£¬
1mol 9mol 6mol 6mol
0.5mol 4.5mol 3mol 3mol
µ±Éś³É3molCO2ŗĶH2OŹ±£¬ŠčŅŖ0.5molC6H12£¬m£ØC6H12£©=0.5mol”Į84g/mol=42g£¬
ŠčŅŖŃõĘųµÄĢå»żĪŖV£ØO2£©=4.5mol”Į22.4L/mol=100.8L£¬
¹Ź“š°øĪŖ£ŗ42£»100.8£»
£Ø3£©ČōĢžA²»ÄÜŹ¹äåĖ®ĶŹÉ«£¬ĖµĆ÷ÓŠ»śĪļÖŠ²»ŗ¬C=C¹ŁÄÜĶÅ£¬µ«ŌŚŅ»¶ØĢõ¼žĻĀ£¬ÄÜÓėĀČĘų·¢ÉśČ”“ś·“Ó¦£¬ĘäŅ»ĀČ“śĪļÖ»ÓŠŅ»ÖÖ£¬Ó¦ĪŖ»·¼ŗĶ飬½į¹¹¼ņŹ½ĪŖ £¬¹Ź“š°øĪŖ£ŗ
£¬¹Ź“š°øĪŖ£ŗ £»
£»
£Ø4£©ČōĢžAÄÜŹ¹äåĖ®ĶŹÉ«£¬ŌŚ“߻ƼĮ×÷ÓĆĻĀÓėH2¼Ó³ÉÉś³É £¬ĖµĆ÷·Ö×ÓÖŠŗ¬ÓŠ1øöC=C¼ü£¬ĻąĮŚĮ½øöĢ¼Ō×Ó¶¼ŗ¬ÓŠHŌ×ÓĪŖC=CĖ«¼üĪ»ÖĆ£¬¹ŹAµÄ½į¹¹¼ņŹ½ĪŖ£ŗ£ØCH3£©3CCH=CH2£¬
£¬ĖµĆ÷·Ö×ÓÖŠŗ¬ÓŠ1øöC=C¼ü£¬ĻąĮŚĮ½øöĢ¼Ō×Ó¶¼ŗ¬ÓŠHŌ×ÓĪŖC=CĖ«¼üĪ»ÖĆ£¬¹ŹAµÄ½į¹¹¼ņŹ½ĪŖ£ŗ£ØCH3£©3CCH=CH2£¬
¹Ź“š°øĪŖ£ŗ£ØCH3£©3CCH=CH2£»
£Ø5£©ČōĢžAÄÜŹ¹äåĖ®ĶŹÉ«£¬ĒŅ·Ö×ÓÖŠĖłÓŠĢ¼Ō×Ó¹²Ę½Ćę£¬ŌņC=CĖ«¼üÖŠ²»±„ŗĶCŌ×ÓĮ¬½ÓĖÄøö¼×»ł£¬¹ŹAµÄ½į¹¹¼ņŹ½ĪŖ£ŗ£ØCH3£©2C=C£ØCH3£©2£¬
¹Ź“š°øĪŖ£ŗ£ØCH3£©2C=C£ØCH3£©2£»
£Ø6£©±ČAÉŁĮ½øöĢ¼Ō×ÓµÄAµÄĻ©ĢžĶ¬ĻµĪļ·Ö×ÓŹ½ĪŖC4H8£¬¶”Ļ©µÄĶ¬·ÖŅģ¹¹ĢåÓŠ£ŗCH2=CHCH2CH3”¢CH3CH=CHCH3”¢CH2=C£ØCH3£©2£¬×ÜÓŠ3ÖÖ¶”Ļ©µÄĶ¬·ÖŅģ¹¹Ģ壬
¹Ź“š°øĪŖ£ŗ3£®
µćĘĄ ±¾Ģāæ¼²éÓŠ»śĪļµÄĶʶĻ£¬Éę¼°·Ö×ÓŹ½”¢½į¹¹¼ņŹ½µÄČ·¶Ø£¬ĢāÄæÄѶČÖŠµČ£¬ĪŖøßĘµæ¼µć£¬°ŃĪÕ¹ŁÄÜĶÅÓėŠŌÖŹµÄ¹ŲĻµĪŖ½ā“šµÄ¹Ų¼ü£¬²ąÖŲÓŠ»śĪļµÄ½į¹¹µÄ·ÖĪöÓėĶʶĻÄÜĮ¦µÄ漲飮


 Ķ¬²½Į·Ļ°Ēæ»ÆĶŲÕ¹ĻµĮŠ“š°ø
Ķ¬²½Į·Ļ°Ēæ»ÆĶŲÕ¹ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
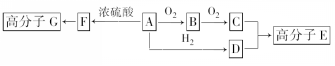
 £®
£® +2H2O£®
+2H2O£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ${\;}_{6}^{14}$CŗĶ${\;}_{6}^{12}$CŹĒĶ¬Ņ»ÖÖŗĖĖŲ | |
| B£® | ŗģĮ×ŗĶ°×Į×»„ĪŖĶ¬ĖŲŅģŠĪĢå | |
| C£® | CH3COOCH2CH3ŗĶCH3CH2COOCH3ŹĒ²»Ķ¬ĪļÖŹ | |
| D£® | CH3CH2OHæÉæ“³ÉŹĒÓÉ-C2H5ŗĶ-OH×é³É |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
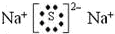 £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŗ£ŃóŌ¼Õ¼µŲĒņ±ķĆ껿µÄ71%£¬ĖłŅŌµŲĒņÉĻ²»Č±Ė® | |
| B£® | ŗ£Ė®µ»ÆÕōĮó·ØµÄ³É±¾×īµĶ | |
| C£® | ŗ£Ė®µ»ÆµÄÖ÷ŅŖ·½·ØÓŠÕōĮó·Ø”¢µēÉųĪö·ØŗĶĄė×Ó½»»»·ØµČ | |
| D£® | ŅŌÉĻĖµ·Ø¶¼ÕżČ· |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | “ĪĀČĖįµÄµēĄė·½³ĢŹ½ĪŖ£ŗHClOØTH++ClO- | |
| B£® | c£ØH+£©µČÓŚ1”Į10-7mol•L-1µÄČÜŅŗŅ»¶ØŹĒÖŠŠŌČÜŅŗ | |
| C£® | ŌŚCH3COONaČÜŅŗÖŠ£¬c£ØCH3COO-£©£¼c£ØNa+£© | |
| D£® | 0.2mol•L-1CH3COOHČÜŅŗÖŠµÄc£ØH+£©ŹĒ0.1mol•L-1 HClČÜŅŗÖŠµÄc£ØH+£©µÄ2±¶ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ź³ŃĪ”¢µĖ® | B£® | ĀČ”¢ä唢µā | C£® | ÄĘ”¢Ć¾”¢ĀĮ | D£® | ÉÕ¼ī”¢ĒāĘų |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com