
��ҵ�����������ᣨ�е㣺90oC��ʱ��ͬʱ�������������ƣ��乤���������£�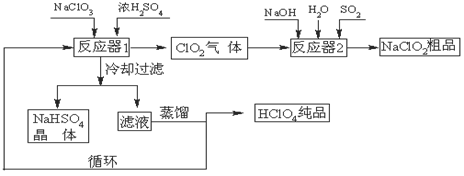
��1����ȴ���˵�Ŀ���ǽ���NaHSO4�� ���������NaHSO4���塣
��2����Ӧ��2�з�����Ӧ�����ӷ���ʽΪ ��SO2��
�������� ����
��3��������ҵ����������Ļ�ѧ��ӦΪ:3NaClO3+3H2SO4��Ũ����3NaHSO4+HClO4+2ClO2+H2O�����������뻹ԭ��������ʵ���֮��Ϊ ��
��4������ͨ��������Һ�ķ����õ��������ԭ������Ǹ�����ķе�Ƚ� ����ߡ��͡��������״���Һ���ݳ���ѭ��ʹ�õ������� ��
��1���ܽ�� ��2��2ClO2��SO2��4OH����2ClO2����SO42����2H2O�� ��ԭ
��3��1��2 ��4���� H2SO4
���������������1���ڷ�Ӧ��1�У������ƺ����ᷴӦ��õ��������Ƶ��ܽ�������¶ȵĽ��Ͷ���С��������ȴ���ˣ����Խ���NaHSO4���ܽ�Ȳ������NaHSO4���塣
��2���ڷ�Ӧ��2�У�����ʵ�ֶ���������NaClO2��ת�����������������Ϊ��ԭ����ClO2��ԭΪNaClO2����Ӧ�����ӷ���ʽΪ2ClO2��SO2��4OH����2ClO2����SO42����2H2O��
��3�����ݷ���ʽ3NaClO3+3H2SO4��Ũ����3NaHSO4+HClO4+2ClO2+H2O��֪��NaClO3������������Ҳ�ǻ�ԭ����������Ԫ�صĻ��ϼ۴ӣ�5�۲������ߵ���7�ۣ����ֽ��͵���4�ۣ����Ը�����������������������ǻ�ԭ������ݵ��ӵ�ʧ�غ��֪�����������뻹ԭ��������ʵ���֮��Ϊ1:2��
��4������ͨ��������Һ�ķ����õ������ᣬ��˵��������ķе�Ƚϵͣ��е㣺90oC��������ѭ��ͼ���Է���������Ϊ��Ӧ����뷴Ӧ��1�У�����Ϊ�������ڷ�Ӧ��2�����ɣ�����ѭ��ʹ�á�
���㣺����������Ʊ���ʵ����������۵��й��жϺͼ���


 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д� �»����ܶ�Ա��ϵ�д�
�»����ܶ�Ա��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�Ӻ�ˮ��ȡþ������������£���ش�������⡣
��1���Ӻ�ˮ����ȡþ����������ͼ��ʾ����ͼ������Ҫ�����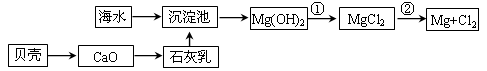
i.��ˮ���������ǰ���Ժ�ˮ���д������������ַ�����
����һ����ɹ�κ��±ˮͨ������أ�
������������������Ũ����ĺ�ˮͨ������ء�
��������________��������������_________________________________________��
ii.��Ӧ�ٵ����ӷ�����________________________________________________��
��Ӧ�ڵĻ�ѧ����ʽ��_______________________________________________��
��2���Ӻ�ˮ����ȡ�����������ͼ��ʾ����ͼ������Ҫ�����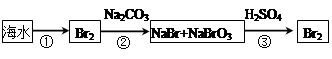

i.���̢��У�������Լ���___________��
ii.���̢��У�����Һ�д����ȿ��������崵�����ô������գ������ȿ�����Ŀ����______________________________________________________________________��
iii.���̢��з�Ӧ�Ļ�ѧ����ʽ��____________________________________________��
iv.�����յõ����嵥������Ȼ����������Cl2�����ȥ�����ʵķ�����__________________________________________________��������ӷ���ʽ�ش𣩡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������ƣ�Na2S2O5��������ʳƷƯ�������Ʊ������������£�
��֪����Ӧ�����2NaHSO3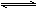 Na2S2O5��H2O�ȶಽ��Ӧ��
Na2S2O5��H2O�ȶಽ��Ӧ��
��1��ʵ������ȡ�����Ļ�ѧ����ʽ�� ��
��2�������ա�ʱ������Ӧ�Ļ�ѧ����ʽ�� ��
��3����֪Na2S2O5��ϡ���ᷴӦ�ų�SO2�������ӷ���ʽΪ�� ��
��4������ƷX�Ļ�ѧʽ�ǣ� ����ѭ�����õ������ǣ�_________��_______��
��5��Ϊ�˼��ٲ�ƷNa2S2O5�����ʺ���������Ʒ�Ӧ�����������������ʵ���֮��ԼΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���绷�����˽���ȫ���ֹʹ��������������ˮ����������������ø�Ч����ɫ���������������ȡ�����������һ�ּ��ױ�ը��ǿ���������壬������ˮ�����ȶ����ʻ���ɫ����������ʹ��ʱ���뾡����ϡ
���������ϡ�ͣ�ͬʱ��Ҫ������ա�����ȡ�ʵ�����Ե�ⷨ�Ʊ�ClO2���������£�
��1��ClO2������ԭ��_____________����ǡ����ǡ���������8���ӽṹ����ͼ��ʾ��ⷨ�ƵõIJ�������������B��ʹʯ����Һ����ɫ����ȥ���������ѡ��_________
A������ʳ��ˮ B����ʯ�� C��Ũ���� D������ˮ
��2���ȶ��Զ���������Ϊ�ƹ�������ȶ����������Ͳ�Ʒ������˵����ȷ����( )
A���������ȿɹ㷺���ڹ�ҵ������ˮ����
B���ȶ��Զ������ȵij��ִ�������˶������ȵ�ʹ�÷�Χ
C���ڹ������ͳ�Ʒ�������ڣ�Ҫ��ͨ��װ�úͼ�⼰����װ��
��3��ŷ������Ҫ��������������Ũ�����Ʊ�����ѧ��Ӧ����ʽΪ_____________________________��ȱ����Ҫ�Dz��ʵ͡���Ʒ���Է��룬��������Ⱦ������
��4���ҹ��㷺���þ��������ϡ�͵�����������������ƣ�NaClO2����Ӧ�Ʊ�����ѧ����ʽ�� ___________________���˷����ŷ�������ŵ���____________________________��
��5����ѧ�����о�����һ���µ��Ʊ����������������ữ�IJ��ᣨH2C2O4����Һ��ԭ����
�ƣ���ѧ��Ӧ����ʽΪ______________________________________________________��
�˷���������������桢����İ�ȫ�ԣ�ԭ���� _________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ҹ����Ԫ�أ����۵���ߵĽ������㷺�������Ƶ��ݵĵ�˿���С�����ʹ�ߡ���������������Ȼ����Ҫ���٣�+6�ۣ����ε���ʽ���ڡ��п��ɼ�ֵ���ٿ�ʯ�ǰ��ٿ�ͺ��ٿ��ٿ����Ҫ�ɷ�������ƣ�CaWO4�������ٿ����Ҫ�ɷ��������̵������Σ���ѧʽ��д�ɣ�Fe��Mn��WO4�����ٿ�ͳұ�����յĵ�һ���Ǽ��۷���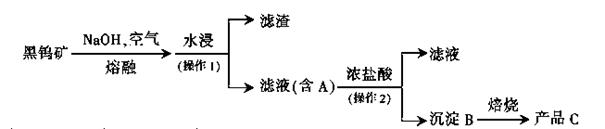
����A��B��C�����ٵĻ�����ش�
��1������ʱ����������ת��Ϊ�������������ƣ�д����Ӧ��Ӧ�Ļ�ѧ����ʽ____��
��2������2�������� ��ʵ������֤������B�Ƿ�ϴ���ķ����� ��ʵ�����б�����Ҫ����Ҫ������____��
��3��д����������ԭ��������ȡ�����ٵĻ�ѧ����ʽ�� ��Ϊ�˻�ÿ������Ƶ�˿�ĸߴ��Ƚ����٣�������̼����������������ԭ������Ϊ____��
��4��ij����ɫ�����ٵĻ�ѧʽ���Ա�ʾΪWO2.8��һ����Ϊ����ɫ�����ٵ���ɫ�ͷ����Ȱ�ʾ���ڻ������д�����ۺ��������ּ�̬���١�����ɫ����������������������ٵ�ԭ����Ŀ֮��Ϊ ��_ ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ���þ���þ��������MgO��KCl��MgCl2��BaCl2��CaCl2��FeCl3�����ʣ�����MgCl2�Ĺ�ҵ�������£�
��֪��25��ʱ�й����ʵ��ܶȻ����£�
| ���� | CaCO3 | MgCO3 | BaCO3 | Mg(OH)2 | Fe (OH)3 |
| Ksp | 4.96��10��9 | 6.82��10��6 | 5.1��10��9 | 5.61��10��12 | 2.64��10��38 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������Ҫ�ɷ�ΪFeTiO3���ɱ�ʾΪFeO��TiO2������������MgO��CaO��SiO2�����ʡ������������Ʊ�����ӵ�ص缫���ϣ������Li4Ti5O12�����������LiFePO4���Ĺ�ҵ��������ͼ��ʾ�� 
��֪��FeTiO3�����ᷴӦ�����ӷ���ʽΪ��FeTiO3��4H+��4Cl-��Fe2+��TiOCl42-��2H2O
��1��������FeTiO3����Ԫ�صĻ��ϼ��� ��
��2������A�ijɷ��� ��
��3����ҺB��TiOCl42- ת������TiO2�����ӷ���ʽ�� ��
��4����Ӧ���й���TiO2ת����(NH4)2Ti5O15��Һʱ��TiԪ�صĽ������뷴Ӧ�¶ȵĹ�ϵ����ͼ��ʾ����Ӧ�¶ȹ���ʱ��TiԪ�ؽ������½���ԭ���� ��
��5����Ӧ�۵Ļ�ѧ����ʽ�� ��
��6������ҺD�Ʊ�LiFePO4�Ĺ����У�����17%˫��ˮ��H2C2O4���������� ��
��7������������ﮣ�Li4Ti5O12������������ﮣ�LiFePO4�����缫��ɵ�أ��乤��ԭ��Ϊ��Li4Ti5O12��3LiFePO4 Li7Ti5O12��3FePO4
Li7Ti5O12��3FePO4
�õ�س��ʱ������Ӧʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������Ĺ�ҵ�Ʒ��У���������������˵��������������Ҫԭ����߶���ȷ����( )
| A������������ǰ��Ҫ�������飬��Ϊ������������ȼ�� |
| B���ӷ���¯������¯���辻������Ϊ¯����SO2�������ʷ�Ӧ |
| C��SO2����ΪSO3ʱ��ʹ�ô����������������SO2��ת���� |
| D��SO3����������Ϊ98%��ŨH2SO4���գ�Ŀ���Ƿ�ֹ�γ��������Ա�ʹSO3������ȫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���й��ڹ�ҵ�����������д������(����)��
| A������ͨ��������Ҫԭ���Ǵ��ʯ��ʯ��ʯӢɰ |
| B����ҵ�ϵ�����ڵ��Ȼ�������ȡ�� |
| C������������������������������������ˮ�����Ƶ�Ũ���� |
| D��������ͨˮ�����Ҫԭ���������ʯ��ʯ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com