
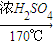 CH2=CH2��+H2O��
CH2=CH2��+H2O�� CH2=CH2��+H2O��
CH2=CH2��+H2O��

 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ŨH2SO4 |
| 170�� |
| ŨH2SO4 |
| 170�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
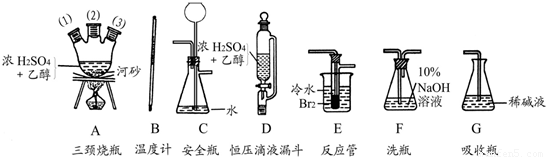
(1)��������������������������Ϊԭ���Ʊ�1��2-�������顣�����������Ϊ�����ң���ȷ������˳����(�̽ӿڻ���Ƥ�ܾ�����ȥ)��___________��A(1)����A�У�__________��A(2)��A(3)��________��________��_________��_________(���д��ĸ����)��

(2)��������ƿA�е���Ҫ��Ӧ�Ļ�ѧ����ʽΪ______________________________________��
(3)�ڷ�Ӧ��E�н��е���Ҫ��Ӧ�Ļ�ѧ����ʽΪ____________________________________��
(4)�¶ȼ�ˮ�������ȷλ����_________________��
(5)��Ӧ��E�м�������ˮ���ѷ�Ӧ��E����ʢ����ˮ��С�ձ�������Ϊ_________________��
(6)Dװ�á���ѹ��Һ©��������������֮���һ�ε��ܵ�������_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ�人���и߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��ʵ���������Ҵ���Ũ���ᷴӦ������ϩ������������ϩ��Ӧ����1,2���������飬���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������140����ˮ�������ѡ��й������б����£�
| | �Ҵ� | 1,2�������� | ���� |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g��cm��3 | 0.79 | 2.2 | 0.71 |
| �е�/�� | 78.5 | 132 | 34.6 |
| �۵�/�� | ��130 | 9 | ��116 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��ʵ���������Ҵ���Ũ���ᷴӦ������ϩ������������ϩ��Ӧ����1,2���������飬���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������140����ˮ�������ѡ��й������б����£�
|
|
�Ҵ� |
1,2�������� |
���� |
|
״̬ |
��ɫҺ�� |
��ɫҺ�� |
��ɫҺ�� |
|
�ܶ�/g��cm��3 |
0.79 |
2.2 |
0.71 |
|
�е�/�� |
78.5 |
132 |
34.6 |
|
�۵�/�� |
��130 |
9 |
��116 |
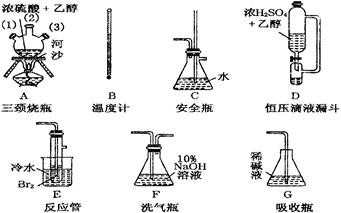
��1�������������Ʊ�1,2���������顣�����������Ϊ�����ң�����ȷ������˳���ǣ��̽ӿڻ���Ƥ�ܾ�����ȥ������������A��1������A�У�������A��2����A��3�������������������������������д��ĸ���ţ���

��2���¶ȼ�ˮ�������ȷλ����������������������
a��֧�ܿڴ� b��Һ���Ϸ�������������c��Һ������
��3���жϸ��Ʊ���Ӧ�Ѿ����������������������������
��4����1,2����������ֲ�Ʒ���ڷ�Һ©����ˮ�����ã�����Ӧ�����������㣨��ϡ������¡�����
��5����������������δ��Ӧ��Br2�������������ϴ�ӳ�ȥ��������ȷѡ��ǰ����ĸ��
a��ˮ����������b������������Һ������c���⻯����Һ��������d���Ҵ�
��6�������������������������ѣ����������������ķ�����ȥ��
��7����Ӧ������Ӧ����ˮ��ȴװ��E������ҪĿ���������������������������ֲ��ܹ�����ȴ�����ñ�ˮ������ԭ������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������һ��2010��������ѧ�ڿ�ǰģ�����ۻ�ѧ ���ͣ�ʵ����
��15�֣�ʵ���������Ҵ���Ũ���ᷴӦ������ϩ��������ȡ1,2���������顣
��1���������������̽ӿڻ���Ƥ�ܾ�����ȥ����ɸ�ʵ�飬����������Ϊ�����ң�����ȷ������˳���ǣ�
B��A��1������A��D��A��2����A��3����______��______��______��______��

��2���¶ȼ�ˮ�������ȷλ����_______________________________��
��3��D���Һ©����ȣ�����Ҫ�ŵ���________________________________��
��4��Eװ���ձ��е���ˮ�ͷ�Ӧ����Һ���ϵ�ˮ�����þ���________________������װ��F�������E�е���Ҫ����ӦΪ_________________________________��
��5����Ҫȷ�ⶨ��ϩ�IJ��������з������е���_____________________��
����I����E��������װ�ò��������ͼ28��1��ʾװ�ý���ʵ�飬��Ӧ��������ʵ��ǰ����������ɴ˵õ���ϩ������
 ����II����E��������װ�ò��������ͼ28��2��ʾװ�ý���ʵ�飬��Ӧ���������ϩ��������ɴ������ϩ������
����II����E��������װ�ò��������ͼ28��2��ʾװ�ý���ʵ�飬��Ӧ���������ϩ��������ɴ������ϩ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com