���㣺�йػ���ﷴӦ�ļ���,���ӷ���ʽ���йؼ���
ר�⣺
����������Ũ�ȡ����һ�������кϽ�����С�����кϽ��������Ҽ��������������С���������������˵���������������������ȫ��Ӧ�����кϽ�����С�ڱ��кϽ����������ҡ����������������ȣ�˵���ҡ�����������ȫ��Ӧ������336mL������Ҫ����������Ϊ510mg��
=612mg��765mg�������н���ʣ�࣬����㣬
��1���ҡ�����������ȫ�����Ը��ݷ�Ӧ�����������������������ʵ���Ũ�ȣ�����n=
�������������ʵ�����������Ԫ���غ��֪n��HCl��=2n��H
2�����ݴ˼��㣻
��2������������ʣ�࣬������ȫ��Ӧ����ʱ��������280mL���ʿ��Ը��ݼ������ݼ�����������ʵ���֮�ȣ���þ���������ʵ����ֱ�Ϊxmol��ymol�����ݶ�������֮�������ת���غ��з��̼���x��y��ֵ���ݴ˽��
��3������ʵ����������м���һ������NaOH��Һ��ǡ��ʹ��Ԫ��ȫ����AlO
2-��ʽ���ڣ���ʹMg
2+�պó�����ȫ����ʱ��Һ������ΪNaCl��NaAlO
2�������������غ���n��NaOH��=n��NaCl��+n��NaAlO
2�������Cl�����ӡ�Alԭ���غ��֪n��NaCl����n��NaAlO
2�����ٸ���V=
������Ҫ����������Һ�������ϣ�1����2���е����ݼ��㣮
���
�⣺����Ũ�����һ�������кϽ�����С�����кϽ��������Ҽ��������������С���������������˵���������������������ȫ��Ӧ�����кϽ�����С�ڱ��кϽ����������ҡ����������������ȣ�˵���ҡ�����������ȫ��Ӧ������336mL������Ҫ����������Ϊ510mg��
=612mg��765mg�������н���ʣ�࣬����㣬
��1���ҡ�����������ȫ�����Ը��ݷ�Ӧ�����������������������ʵ���Ũ�ȣ�������ȫ��Ӧ��������672mL�����������ʵ���Ϊ��
=0.03mol��������Ԫ���غ��֪n��HCl��=2n��H
2��=2��0.03mol=0.06mol��
����������ʵ���Ũ��Ϊ��
=2mol/L��
�ʴ�Ϊ��2mol/L��
��2������������ʣ�࣬������ȫ��Ӧ����ʱ��������280mL���ʿ��Ը��ݼ������ݼ�����������ʵ���֮�ȣ���þ���������ʵ����ֱ�Ϊxmol��ymol�����ݶ���������֪����24x+27y=0.51�����ݵ���ת���غ��У���2x+3y=
��2=0.05��
�٢��������̽�ã�x=0.01��y=0.01��
�ʺϽ���þ����������Ϊ��
��100%=47.1%��
�𣺺Ͻ���þ����������Ϊ47.1%��
��3������ʵ����������м���һ������NaOH��Һ��ǡ��ʹ��Ԫ��ȫ����AlO
2-��ʽ���ڣ���ʹMg
2+�պó�����ȫ����ʱ��Һ������ΪNaCl��NaAlO
2�������������غ㣬��n��NaOH��=n��NaCl��+n��NaAlO
2�������Cl�����ӡ�Alԭ���غ㣬��֪n��NaOH��=n��NaCl��+n��NaAlO
2��=n��HCl��+n��Al��=0.06mol+0.01mol=0.07mol��
����Ҫ1mol/L NaOH��Һ�����Ϊ��
=0.07L=70mL��
�𣺼���ʵ������������м���lmol?L
-1��NaOH��Һ70����ǡ��ʹ��Ԫ����[Al��OH��
4]
-���ڣ���ʹMg
2+�պó�����ȫ��
���������⿼������ļ��㣬��Ŀ�Ѷ��еȣ����ݱ������ݹ�ϵ�жϷ�Ӧ�Ĺ��������ǹؼ���ע�������йػ�������ķ����뼼�ɣ�������ؿ���ѧ�������ݵķ���������������������Ŀ��飮



 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
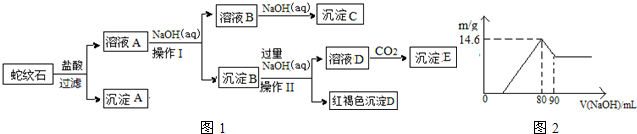
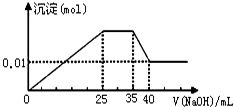 ��һδ֪����ɫ��Һ��ֻ���ܺ������������е������֣�������ˮ���������H+��OH-����H+��NH4+��K+��Mg2+��Cu2+��Al3+��NO3-��CO32-��SO42-����ȡ����100mL��Һ��������ʵ�飺
��һδ֪����ɫ��Һ��ֻ���ܺ������������е������֣�������ˮ���������H+��OH-����H+��NH4+��K+��Mg2+��Cu2+��Al3+��NO3-��CO32-��SO42-����ȡ����100mL��Һ��������ʵ�飺