
»ÆŗĻĪļ¢õŹĒÖŠŅ©»ĘÜĖÖŠµÄÖ÷ŅŖ»īŠŌ³É·ÖÖ®Ņ»,¾ßÓŠæ¹Ńõ»ÆŗĶæ¹Ö×Įö×÷ÓĆ”£»ÆŗĻĪļ¢õŅ²æÉĶعżĻĀĶ¼ĖłŹ¾·½·ØŗĻ³É:

(1)»ÆŗĻĪļ¢ńµÄ·Ö×ÓŹ½ĪŖ””””””””,¢õÖŠŗ¬ÓŠµÄŗ¬Ńõ¹ŁÄÜĶŵÄĆū³ĘĪŖ”””””””””£
(2)»ÆŗĻĪļ¢ņµÄŗĻ³É·½·ØĪŖ
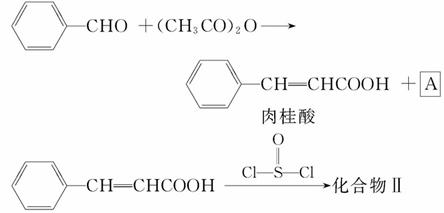
ŗĻ³ÉČā¹šĖįµÄ·“Ó¦·½³ĢŹ½ÖŠ,·“Ó¦ĪļµÄĪļÖŹµÄĮæÖ®±ČĪŖ1”Ć1,Éś³ÉĪļA³ŹĖįŠŌ,ŌņĘäĆū³ĘŹĒ”””””””””£Ņ»¶ØĢõ¼žĻĀ,Čā¹šĖįÓėŅŅ“¼·“Ӧɜ³ÉĻćĮĻČā¹šĖįŅŅõ„,Ęä·“Ó¦·½³ĢŹ½ĪŖ ””(²»ŅŖĒó±ź³ö·“Ó¦Ģõ¼ž)”£
(3)ĻĀĮŠ¹ŲÓŚ»ÆŗĻĪļ¢õµÄĖµ·ØÕżČ·µÄŹĒ”””” ””””(²»¶ØĻīŃ”Ōń,Ģī×ÖÄø)”£
A.·Ö×ÓÖŠÓŠČżøö±½»· B.ÄÜ·¢ÉśĖ®½ā·“Ó¦
C.Ź¹ĖįŠŌKMnO4ČÜŅŗĶŹÉ« D.ÓėFeCl3·¢ÉśĻŌÉ«·“Ó¦
(1)øł¾Ż»ÆŗĻĪļ¢ńµÄ½į¹¹¼ņŹ½æÉČ·¶ØĘä·Ö×ÓŹ½ĪŖC9H12O4;¢õÖŠŗ¬ÓŠµÄŗ¬Ńõ¹ŁÄÜĶÅ°üĄØōĒ»ł”¢ĆŃ¼ü”¢ōŹ»ł”£
(2)øł¾Ż»ÆŗĻĪļ¢ņµÄŗĻ³É·½·Ø·ÖĪö,Éś³ÉĪļAµÄ·Ö×ÓŹ½ĪŖ(øł¾ŻŌ×ÓŹŲŗć)C2H4O2,ÓÖŅņA³ŹĖįŠŌ,æÉČ·¶ØAĪŖCH3COOH,¼“ŅŅĖį»ņ“×Ėį”£
(3)»ÆŗĻĪļ¢õÖŠÖ»ÓŠĮ½øö±½»·,²»ÄÜ·¢ÉśĖ®½ā·“Ó¦,ÓÉÓŚ·ÓōĒ»łŗĶĢ¼Ģ¼Ė«¼üµÄ“ęŌŚ,æÉŅŌ±»ĖįŠŌKMnO4ČÜŅŗŃõ»Æ,Ķ¬Ź±·ÓōĒ»łŅ²æÉŅŌÓėFeCl3·¢ÉśĻŌÉ«·“Ó¦”£
“š°ø:(1)C9H12O4 ōĒ»ł”¢ĆŃ¼ü”¢ōŹ»ł
(2)ŅŅĖį(»ņ“×Ėį)
 +C2H5OH
+C2H5OH
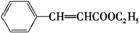 +H2O
+H2O
(»ņC6H5CH£½CHCOOH+C2H5OH C6H5CH£½CHCOOC2H5+H2O)
C6H5CH£½CHCOOC2H5+H2O)
(3)CӢD


 æĪæĪĶØæĪ³Ģ±ź×¼Ė¼Ī¬·½·ØÓėÄÜĮ¦ŃµĮ·ĻµĮŠ“š°ø
æĪæĪĶØæĪ³Ģ±ź×¼Ė¼Ī¬·½·ØÓėÄÜĮ¦ŃµĮ·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ē°ĖÄÖÜĘŚŌŖĖŲÖŠ£¬»łĢ¬Ō×ÓÖŠĪ“³É¶Ōµē×ÓŹżÓėĘäĖłŌŚÖÜĘŚŹżĻąĶ¬µÄŌŖĖŲ¹²ÓŠ £Ø £©
A£®6ÖÖ B£®5ÖÖ C£®4ÖÖ D£®3ÖÖ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Š“³öĻĀĮŠŌŚÉś²ś”¢Éś»īÓ¦ÓĆÖŠĘųĢåµÄ²śÉśŌĄķ(ÓĆ»Æѧ·½³ĢŹ½±ķŹ¾)£ŗ
(1)Ē±Ė®Ķ§ŗĶĻū¶¾ŗōĪüĆę¾ßÖŠ²śÉśO2£ŗ__________________________________________”£
(2)ÅŻÄĆš»šĘ÷ÓĆÓŚĆš»šŹ±²śÉśCO2£ŗ____________________________________________”£
(3)ÖʱøĻū¶¾ĘųCl2£¬ÓĆKMnO4“śĢęMnO2£ŗ_______________________________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Įņ“śĮņĖįÄĘŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤²śĘ·”£Ä³ŠĖȤŠ”×éÄāÖʱøĮņ“śĮņĖįÄĘ¾§Ģå(Na2S2O3·5H2O)”£
¢ń.[²éŌÄ׏ĮĻ]
(1)Na2S2O3·5H2OŹĒĪŽÉ«ĶøĆ÷¾§Ģ壬Ņ×ČÜÓŚĖ®£¬ĘäĻ”ČÜŅŗÓėBaCl2ČÜŅŗ»ģŗĻĪŽ³ĮµķÉś³É”£
(2)ĻņNa2CO3ŗĶNa2S»ģŗĻČÜŅŗÖŠĶØČėSO2æÉÖʵĆNa2S2O3£¬ĖłµĆ²śĘ·³£ŗ¬ÓŠÉŁĮæNa2SO3ŗĶNa2SO4”£
(3)Na2SO3Ņ×±»Ńõ»Æ£»BaSO3ÄŃČÜÓŚĖ®£¬æÉČÜÓŚĻ”HCl”£
¢ņ.[Öʱø²śĘ·]
ŹµŃé×°ÖĆČēĶ¼ĖłŹ¾(Ź”ĀŌ¼Š³Ö×°ÖĆ)£ŗ
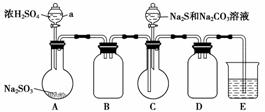
ŹµŃé²½Öč£ŗ
(1)¼ģ²é×°ÖĆĘųĆÜŠŌ£¬°“Ķ¼Ź¾¼ÓČėŹŌ¼Į”£
ŅĒĘ÷aµÄĆū³ĘŹĒ________£»EÖŠµÄŹŌ¼ĮŹĒ________(Ń”ĢīĻĀĮŠ×ÖÄø±ąŗÅ)”£
A£®Ļ”H2SO4
B£®NaOHČÜŅŗ
C£®±„ŗĶNaHSO3ČÜŅŗ
(2)ĻČĻņCÖŠÉÕĘæ¼ÓČėNa2SŗĶNa2CO3»ģŗĻČÜŅŗ£¬ŌŁĻņAÖŠÉÕĘæµĪ¼ÓÅØH2SO4”£
(3)“żNa2SŗĶNa2CO3ĶźČ«ĻūŗÄŗ󣬽įŹų·“Ó¦”£¹żĀĖCÖŠ»ģŗĻĪļ£¬ĀĖŅŗ¾__________(ĢīŠ“²Ł×÷Ćū³Ę)”¢½į¾§”¢¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ£¬µĆµ½²śĘ·”£
¢ó.[Ģ½¾æÓė·“Ė¼]
(1)ĪŖŃéÖ¤²śĘ·ÖŠŗ¬ÓŠNa2SO3ŗĶNa2SO4£¬øĆŠ”×éÉč¼ĘĮĖŅŌĻĀŹµŃé·½°ø£¬Ēė½«·½°ø²¹³äĶźÕū”£
(ĖłŠčŹŌ¼Į“ÓĻ”HNO3”¢Ļ”H2SO4”¢Ļ”HCl”¢ÕōĮóĖ®ÖŠŃ”Ōń)
Č”ŹŹĮæ²śĘ·Åä³ÉĻ”ČÜŅŗ£¬µĪ¼Ó×ćĮæBaCl2ČÜŅŗ£¬ÓŠ°×É«³ĮµķÉś³É£¬________________£¬Čō³ĮµķĪ“ĶźČ«Čܽā£¬²¢ÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄĘųĢå²śÉś£¬ŌņæÉČ·¶Ø²śĘ·ÖŠŗ¬ÓŠNa2SO3ŗĶNa2SO4”£
(2)ĪŖ¼õɣװÖĆCÖŠÉś³ÉNa2SO4µÄĮ棬ŌŚ²»øıäŌӊװÖƵĻł“”ÉĻ¶ŌŹµŃé²½Öč(2)½ųŠŠĮĖøĽų£¬øĽųŗóµÄ²Ł×÷ŹĒ
________________________________________________________________________ӣ
(3)Na2S2O3·5H2OµÄČܽā¶ČĖęĪĀ¶ČÉżøßĻŌÖųŌö“ó£¬ĖłµĆ²śĘ·Ķعż________________·½·ØĢį“攣
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijæĪĶāŹµŃ銔×éÉč¼ĘµÄĻĀĮŠŹµŃéŗĻĄķµÄŹĒ(””””)
|
|
|
|
|
| A.Öʱø ÉŁĮæ°±Ęų | B.ĪüŹÕ HCl | C.ÅäÖĘŅ»¶Ø ÅضČĮņĖį ČÜŅŗ | D.Öʱø²¢ ŹÕ¼ÆÉŁĮæ NO2ĘųĢå |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
æÉÄę·“Ó¦N2£«3H2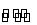 2NH3µÄÕż”¢Äę·“Ó¦ĖŁĀŹæÉÓĆø÷·“Ó¦ĪļŗĶÉś³ÉĪļÅØ¶ČµÄ±ä»ÆĄ“±ķŹ¾”£ĻĀĮŠø÷¹ŲĻµÖŠÄÜĖµĆ÷·“
2NH3µÄÕż”¢Äę·“Ó¦ĖŁĀŹæÉÓĆø÷·“Ó¦ĪļŗĶÉś³ÉĪļÅØ¶ČµÄ±ä»ÆĄ“±ķŹ¾”£ĻĀĮŠø÷¹ŲĻµÖŠÄÜĖµĆ÷·“ Ó¦ŅŃ“ļµ½Ę½ŗāדĢ¬µÄŹĒ( )
Ó¦ŅŃ“ļµ½Ę½ŗāדĢ¬µÄŹĒ( )
A.3vÕż(N2)£½vÕż(H2) B.vÕż(N2)£½vÄę(NH3)
C.2vÕż(H2)£½3vÄę(NH3) D.vÕż(N2)£½3vÄę(H2)
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ(””””)
A.±½ŗĶŅŅĻ©¶¼ÄÜÓėH2·¢Éś¼Ó³É·“Ó¦
B.ÕįĢĒŌŚČĖĢåÄŚĖ®½āµÄ²śĪļÖ»ÓŠĘĻĢŃĢĒ
C.Ź³“×ÖŠŗ¬ÓŠŅŅĖį,ŅŅĖįæÉÓÉŅŅ“¼Ńõ»ÆµĆµ½
D.ĆŗæÉÓėĖ®ÕōĘų·“Ó¦ÖĘ³ÉĖ®ĆŗĘų,Ė®ĆŗĘųµÄÖ÷ŅŖ³É·ÖĪŖCOŗĶH2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ČēĶ¼ĖłŹ¾3Ģ׏µŃé×°ÖĆ£¬·Ö±š»Ų“šĻĀĮŠĪŹĢā£ŗ
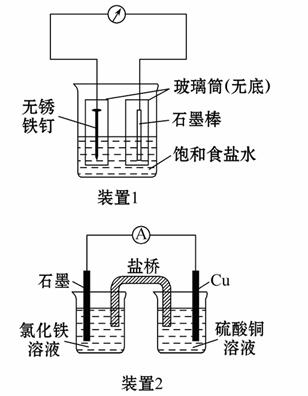
(1)×°ÖĆ1ĪŖĢśµÄĪüŃõøÆŹ“ŹµŃ锣Ņ»¶ĪŹ±¼äŗó£¬Ļņ²åČėĢś¶¤µÄ²£Į§Ķ²ÄŚµĪČėKS CNČÜŅŗ³ŹĪŽÉ«£¬ŌŁµĪČėĀČĖ®¼“æɹŪ²ģµ½Ģś¶¤ø½½üµÄČÜŅŗ±äŗģÉ«£¬±ķĆ÷Ģś±»_______£»Ļņ²åČėĢ¼°ōµÄ²£Į§Ķ²ÄŚµĪČė·ÓĢŖŹŌŅŗ£¬æɹŪ²ģµ½Ģ¼°ōø½½üµÄČÜŅŗ±äŗģ£¬øƵē¼«·“Ó¦ĪŖ_______________”£
CNČÜŅŗ³ŹĪŽÉ«£¬ŌŁµĪČėĀČĖ®¼“æɹŪ²ģµ½Ģś¶¤ø½½üµÄČÜŅŗ±äŗģÉ«£¬±ķĆ÷Ģś±»_______£»Ļņ²åČėĢ¼°ōµÄ²£Į§Ķ²ÄŚµĪČė·ÓĢŖŹŌŅŗ£¬æɹŪ²ģµ½Ģ¼°ōø½½üµÄČÜŅŗ±äŗģ£¬øƵē¼«·“Ó¦ĪŖ_______________”£
(2)×°ÖĆ2ÖŠµÄŹÆÄ«ŹĒ_________¼«(Ģī”°Õż”±»ņ”°øŗ”±)£¬øĆ×°ÖĆ·¢ÉśµÄ×Ü·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ________________”£
(3)×°ÖĆ3ÖŠ¼×ÉÕ±Ź¢·Å100 mL 0.2 mol”¤L-1µÄNaClČÜŅŗ£¬ŅŅÉÕ±Ź¢·Å100 mL 0.5 mol”¤L-1µÄCuSO4ČÜŅŗ”£·“Ó¦Ņ»¶ĪŹ±¼äŗó£¬Ķ£Ö¹Ķصē”£Ļņ¼×ÉÕ±ÖŠµĪČė¼øµĪ·ÓĢŖŹŌŅŗ£¬¹Ū²ģµ½ŹÆÄ«µē¼«ø½½üŹ×Ļȱäŗģ”£
¢ŁµēŌ“µÄM¶ĖĪŖ_________¼«£¬¼×ÉÕ±ÖŠĢśµē¼«µÄµē¼«·“Ó¦ĪŖ________________£»
¢ŚŅŅÉÕ±ÖŠµē½ā·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ___________________________________£»
¢ŪĶ£Ö¹µē½ā£¬Č”³öCuµē¼«£¬Ļ“µÓ”¢øÉŌļ”¢³ĘĮ棬µē¼«ŌöÖŲ0.64 g£¬Ōņ¼×ÉÕ±ÖŠ²śÉśµÄĘųĢåŌŚ±ź×¼×“æöĻĀĪŖ____________ mL”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
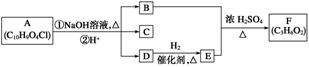
ÓŠ»śĪļA”«FÖ®¼äµÄ×Ŗ»Æ¹ŲĻµČēĶ¼ĖłŹ¾(²æ·Ö²śĪļŅŃĀŌČ„)£¬ĘäÖŠBµÄĻą¶Ō·Ö×ÓÖŹĮæŹĒDµÄ2±¶”£
ĢįŹ¾£ŗ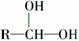 »į×Ō¶ÆĶŃĖ®ŠĪ³ÉR—CHO
»į×Ō¶ÆĶŃĖ®ŠĪ³ÉR—CHO
øł¾ŻŅŌÉĻŠÅĻ¢»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)BµÄ·Ö×ÓŹ½ŹĒ__________________”£
(2)¼ģŃéDÖŠ¹ŁÄÜĶÅæÉŹ¹ÓĆŹŌ¼ĮµÄĆū³ĘŹĒ_________________________________________£¬
Š“³öDµÄĻąĮŚĶ¬ĻµĪļÓėøĆŹŌ¼Į·“Ó¦µÄ»Æѧ·½³ĢŹ½___________________________________
________________________________________________________________________ӣ
(3)CÓöFeCl3ČÜŅŗĻŌ×ĻÉ«£¬ŗĖ“Ź²ÕńĒāĘ×ÖŠÓŠĖÄøö·å£¬Ęä·åĆ껿֮±ČĪŖ1”Ć2”Ć2”Ć1”£Š“³öCµÄ½į¹¹¼ņŹ½__________________________”£
(4)Š“³ö·ūŗĻĻĀĮŠĢõ¼žµÄCµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½________________”¢______________”¢______________”¢______________”£
¢Ł±½»·ÉĻ“ęŌŚ¶ŌĪ»Č”“ś»ł£»
¢ŚÓöFeCl3ČÜŅŗĻŌ×ĻÉ«£»
¢ŪÄÜÓėŅų°±ČÜŅŗ·“Ó¦”£
(5)Š“³öAµÄ½į¹¹¼ņŹ½________________________________________________________”£
(6)Čō1 mol AŗĶ×ćĮæNaOHČÜŅŗ·“Ó¦£¬×ī¶ąÄÜĻūŗÄ______ mol NaOH”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com