

 �������Ʊ�һ����Ҫ���л�����ԭ��ˮ����
�������Ʊ�һ����Ҫ���л�����ԭ��ˮ���� ��ʹ������ͼ��ʾ���Ʊ����̣�������ͼʾ����CH3CH2OH
��ʹ������ͼ��ʾ���Ʊ����̣�������ͼʾ����CH3CH2OH| Ũ���� |
| 170�� |
| Br2 |
| 242��26.45% |
| 16 |
| 242��69.42% |
| 12 |
| 242-4��16-14��12 |
| 1 |
 �Ʊ��л�����ԭ��ˮ����
�Ʊ��л�����ԭ��ˮ����  �����ȷ���������Ӧ����
�����ȷ���������Ӧ����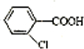 ��Ȼ��ˮ�⡢�ữ�ɵ�
��Ȼ��ˮ�⡢�ữ�ɵ� ��
��| 242��26.45% |
| 16 |
| 242��69.42% |
| 12 |
| 242-4��16-14��12 |
| 1 |
 ��
�� ��
�� �Ʊ��л�����ԭ��ˮ����
�Ʊ��л�����ԭ��ˮ����  �����ȷ���������Ӧ����
�����ȷ���������Ӧ���� ��Ȼ��ˮ�⡢�ữ�ɵ�
��Ȼ��ˮ�⡢�ữ�ɵ� ������Ӧע������ŵ���������Ȱ�-CHO����Ϊ-COOH���������-OH����ֹ-OH����������Ӧ������Ϊ
������Ӧע������ŵ���������Ȱ�-CHO����Ϊ-COOH���������-OH����ֹ-OH����������Ӧ������Ϊ ��
�� ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��ѧʽ | FeS | CuS | MnS |
| �ܶȻ� | 6.3��10-18 | 1.3��10-36 | 2.5��10-13 |
| A��25��ʱ��CuS���ܽ�ȴ���MnS���ܽ�� |
| B��25��ʱ������CuS��Һ�У�Cu2+��Ũ��Ϊ1.3��10-36 mol?L-1 |
| C����ΪH2SO4��ǿ�ᣬ���Է�ӦCuSO4+H2S=CuS��+H2SO4���ܷ��� |
| D����ȥij��Һ�е�Cu2+������ѡ��FeS�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | ��һ�� | �ڶ��� | ������ | ������ |
| t/�� | 30 | 40 | 50 | 80 |
| NH3������/��10-6mol�� | 4.8 | 5.9 | 6.0 | 2.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
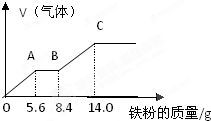 ijϡ�����ϡ����Ļ����Һ100mL���������������ۣ�������������������������ӵı仯��ͼ��ʾ����֪����Ļ�ԭ����ֻ��NO���壩
ijϡ�����ϡ����Ļ����Һ100mL���������������ۣ�������������������������ӵı仯��ͼ��ʾ����֪����Ļ�ԭ����ֻ��NO���壩�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| HCN/OH- |

| H2O/H+ |

 ����������NaOHˮ��Һ�м��ȷ�Ӧʱ�Ļ�ѧ����ʽΪ
����������NaOHˮ��Һ�м��ȷ�Ӧʱ�Ļ�ѧ����ʽΪ �ĺϳ�·������ͼ�����Լ����ã���
�ĺϳ�·������ͼ�����Լ����ã���| HBr |
| NaOH��Һ |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ȥ�����л��е�������ϩ��ͨ����ˮ������ |
| B����ȥ�屽�л��е����������壺����������NaOH��Һ�������á���Һ |
| C����ȥ96%���Ҵ��е�����ˮ����ˮ�Ҵ���������ʯ�ң������á����� |
| D����ȥ�Ҵ��е���ˮ�ɼ�������ƣ�ʹ����ȫ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| �� | CH4 | CH3CH3 | CH3��CH2��2CH3 | �������� |  |  | 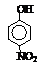 |
| �е�/�� | -164 | -88.6 | -0.5 | �۵�/�� | 45 | 96 | 114 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com