
 ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�
ij������ȤС�����ⶨijNaOH��Һ��Ũ�ȣ�������������£�| �ζ����� | ����Һ�����mL�� | �����������mL�� | |
| �ζ�ǰ������mL�� | �ζ��������mL�� | ||
| ��һ�� | 25.00 | 0.00 | 19.90 |
| �ڶ��� | 25.00 | 0.00 | 20.00 |
| ������ | 25.00 | 4.00 | 24.10 |
���� ��1������Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻
��2�����ݵζ�ʱ�۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯��
��3�����ݴ�����Һ��ϴ��ƿ����ʹ�Ĵ���Һ�����ʵ������࣬����c�����⣩=$\frac{c��������V������}{V�����⣩}$������
��4���ζ���װҺǰ������ʢװ����Һ��ϴ������c�����⣩=$\frac{c��������V������}{V�����⣩}$������
��5�����ݵζ��ܵĽṹ�;�ȷ�ȣ�
��6���ȷ������������Һ���������Ч�ԣ�Ȼ��������������Һ�����ƽ��ֵ��Ȼ��Ȼ����ݹ�ϵʽHCl��NaOH�����
��� �⣺��1���ζ��յ�ʱ����ƿ�е���Һ�Ӻ�ɫ��Ϊ��ɫʱ���Ұ�����ڲ���ɫ��ֹͣ�ζ���
�ʴ�Ϊ���죻�ޣ�
��2���ζ�ʱ�۾�Ӧ�۲���ƿ����Һ��ɫ�ı仯�����ж��յ�ĵ����ѡ��B��
��3�����ݴ�����Һ��ϴ��ƿ����ʹ�Ĵ���Һ�����ʵ������࣬���V������ƫ����c�����⣩=$\frac{c��������V������}{V�����⣩}$������c�����⣩ƫ�ʴ�Ϊ��������Һ��ϴ��ƿ��ƫ�ߣ�
��4������ʽ�ζ���������ˮϴ��������0.1000mol/L��������ϴ2-3�Σ���������ע��0.1000mol/L���������ʽ�ζ���������ˮϴ��������������ע��0.1000mol/L�����ᣬ���ᱻϡ�ͣ�����V������ƫ����c�����⣩=$\frac{c��������V������}{V�����⣩}$������c�����⣩ƫ�ߣ�
�ʴ�Ϊ��δ�ñ�Һ��ϴ��ʽ�ζ��ܣ�ƫ�ߣ�
��5���ζ����е�Һ��Ķ���Ϊ22.60mL��
�ʴ�Ϊ��22.60��
��6�����ݱ������ݣ���һ�εζ����ĵı�Һ���Ϊ��19.90mL���ڶ��εζ����ĵı�Һ���Ϊ��20.00mL�������εζ����ĵı�Һ���Ϊ��20.10mL�����ξ���Ч���ζ����ĵı�Һƽ�����Ϊ��20.00mL
HCl��NaOH
1 1
0.1000mol/L��20.00mL c��NaOH����25.00mL
��� c��NaOH��=0.0800mol/L
�ʴ�Ϊ��0.0800mol/L��
���� ������Ҫ��������к͵ζ�����ȷ�к͵ζ�ʵ������������衢ָʾ����ѡ�����ݴ����ȼ��ɽ����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڶ������У���ۺ�����������ǿ���Ƿ� | |
| B�� | ��ķǽ����Ա����� | |
| C�� | VA����ԭ�Ӱ뾶��С���ǵ� | |
| D�� | �ƵĽ����Ա��ǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
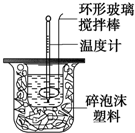 ������ͼװ�òⶨ�кͷ�Ӧ�ķ�Ӧ�ȵ�ʵ�鲽�����£�
������ͼװ�òⶨ�кͷ�Ӧ�ķ�Ӧ�ȵ�ʵ�鲽�����£�| �¶� ʵ������� | ��ʼ�¶�t1�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 29.5 | 3.4 |
| 2 | 25.9 | 25.9 | 25.9 | 29.2 | |
| 3 | 26.4 | 26.2 | 26.3 | 29.8 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������ԭ�Ӷ���ͬһƽ���� | |
| B�� | ������ˮ�����ӳɷ�Ӧʹ����ɫ | |
| C�� | ����ŨHNO3��һ�������·���ȡ����Ӧ | |
| D�� | ��������KMnO4��Һ��Ӧʹ����ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | $\overline{v}$��O2��=0.01mol/��L•s�� | B�� | $\overline{v}$��NO��=0.08mol/��L•s�� | ||
| C�� | $\overline{v}$��H2O��=0.0013mol/��L•s�� | D�� | $\overline{v}$��NH3��=0.002mol/��L•s�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
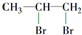 1��2������飻 ��
1��2������飻 ��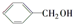 ���״���
���״��� C4H8O�� ��
C4H8O�� ��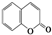 C9H6O2��
C9H6O2���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | һ������ | C�� | һ����̼ | D�� | ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com