
��298K��1.01��105Pa�£���32 g SO2ͨ��750 mL 1 mol��L��1 KOH��Һ�г�ַ�Ӧ����÷�Ӧ�ų�x kJ����������֪�ڸ������£�1 mol SO2ͨ��1 L 2 mol ��L��1KOH��Һ�г�ַ�Ӧ�ų�y kJ���������� SO2��KOH��Һ��Ӧ���� KHSO3���Ȼ�ѧ����ʽ��ȷ���� (����)��
A��SO2(g)��KOH (aq)===KHSO3 (aq)
��H����(4x��y) kJ��mol
B��SO2(g)��KOH (aq)===KHSO3 (aq)
��H����(2x��y) kJ��mol��1
C��SO2(g)��KOH (aq)===KHSO3 (aq)
��H����(2y��x) kJ��mol
D��2SO2(g)��2KOH (l)===2KHSO3 (l)
��H����(8x��2y) kJ��mol��1
������1 mol SO2ͨ��1 L 2 mol��L��1 KOH ��Һ�У�����1 mol K2SO3���ų�
y kJ���������ɴ˿ɵ�SO2(g)��2KOH (aq )===K2SO3 (aq) ��H2O(l)����H��
�� y kJ��mol �١�32 g SO2 ͨ��750 mL 1 mol ��L��1 KOH��Һ�г�ַ�Ӧ������ K2SO3��KHSO3�� 0.25 mol ����÷�Ӧ�ų� x kJ �����������Ȼ�ѧ����ʽΪ 2SO2(g)��3KOH(aq)===K2SO3(aq)��KHSO3(aq)��H2O(l)����H����4x kJ��mol��1���ڡ��ɢڣ��ٿɵ�SO2��KOH��Һ��Ӧ����KHSO3���Ȼ�ѧ����ʽ��
�𰸡�A


 ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����л�����ȫȼ��ʱ����������CO2��H2O�⣬���������������(����)
A����ά�� B����֬
C�������� D��Ӳ֬��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���¼����������£�ij��Ӧ��ƽ�ⳣ��K�� ������ʱ���¶����ߣ�H2Ũ�ȼ�С������˵����ȷ���� (����)��
������ʱ���¶����ߣ�H2Ũ�ȼ�С������˵����ȷ���� (����)��
A�������£������������Ϊԭ��2����CO��ƽ��Ũ�ȱ�Ϊԭ����1/2
B�����º����£�����ѹǿ��H2Ũ��һ����С
C�������¶ȣ�����Ӧ���ʼ�С���淴Ӧ��������
D���÷�Ӧ��ѧ����ʽΪCO2��H2 CO��H2O����H<0
CO��H2O����H<0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ���¶��£���1 mol A��1 mol B�������2 L�ܱ�������������Ӧ��A(g)��B(g)xC(g)��D(s)����H>0��t1ʱ�ﵽƽ�⡣��t2��t3ʱ�̷ֱ�ı䷴Ӧ��һ���������������������C��Ũ����ʱ��仯��ͼ��ʾ������˵����ȷ���� (����)��

A��t1��t3��÷�Ӧ��ƽ�ⳣ����Ϊ4
B����Ӧ����ʽ�е�x��1
C��t2ʱ�̸ı��������ʹ�ô���
D��t3ʱ�̸ı����������ȥ��������D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��Ӧ��������Ӧ A BC���ɣ���Ӧ�����е������仯������ͼ������������ȷ���� (����)��

A��������Ӧ��Ϊ���ȷ�Ӧ
B�����ֻ�������C���ȶ�
C�����������ı䷴Ӧ���ʱ�
D��������Ӧ�Ħ�H��E1��E2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ����Ŀǰȼ��ȼ��ʱ�����Ļ�����Ⱦ��ͬʱ������ԴΣ�����й�ר�����������̫������ȡ���ܵĹ��롣����˵����ȷ���� (����)��
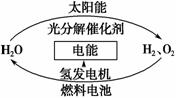
A��H2O�ķֽⷴӦ�Ƿ��ȷ�Ӧ
B������Դ�ѱ��ձ�ʹ��
C��2 mol H2O�������������2 mol H2��1 mol O2������
D������������������䣬�������ü�ֵ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪ H2SO4(aq)�� Ba (OH)2(aq)��Ӧ�� ��H����1 584.2 kJ��mol��1��HCl (aq) ��NaOH (aq) ��Ӧ�� ��H����55.6 kJ��mol��1�������� BaSO4 (s) �ķ�Ӧ�ȵ��� (����)��
A����1 528.6 kJ��mol��1�� B����1 473 kJ��mol��1
C����1 473 kJ��mol��1�� D����1 528.6 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ��ʡҩƷ��ʱ�䣬�ס��ҡ�����λͬѧ��ͭƬ��пƬ��ϡ���ᡢCuSO4 ��Һ��ֱ����Դ��ʯī�缫�����ߡ��ձ����Թܵ���ѧ��ѧ������ҩƷ������(��Ʒ)��ͨ������Ĺ�˼������˱Ƚ�ͭ��п����������ǿ����ϵ��ʵ�顣����д���пհף�
(1)��ͬѧ�ֱ�һСƬͭƬ��пƬ�����ձ��ײ�(ͭ��п���Ӵ�)�����ձ���С�ļ���ϡ���ᣬ�۲쵽��������
________________________________________________________________��
��ͬѧ�����˼·��______________________________________________��
(2)��ͬѧ���ż�ʵ�飬���ձ��еμ�________��Һ�������۲쵽��������______________________________________________________________��
��ͬѧ����п��ͭ����������ǿ���Ľ��������ݵ�ԭ����________________________________________________________________________��
(3)��ͬѧʹ��ֱ����Դ��ʯī�缫��װ�õ��װ�ã�����ͬѧʵ������Һ�в����˱�Ҫ���Լ�________��Һ(��Ϊ���Һ)����Ӧ�ڵ������漴��ʼ��ʵ�����йط�Ӧ�Ļ�ѧ����ʽΪ______________________________________��
ʵ���е�����������______________________________________________��
(4)�����ٵ������һ����ʵ��(�Լ���������ѡ)��̽����֤ʵп��ͭ������Ե����ǿ��(��Ҫ˵������������)___________________________________________________________
_______________________________________________________________
_______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڿ��淴Ӧ�У�ƽ�ⳣ���뷴Ӧ���е��ȵĹ�ϵ��ȷ����(����)
A��KԽ��Ӧ�̶�Խ��
B��KԽ��Ӧ�̶�ԽС
C��K�Ĵ�С�뷴Ӧ�̶���
D�������¶ȣ�K����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com