
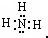
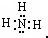
| ||
| ||
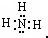 £¬H2OµÄ½į¹¹Ź½ĪŖ£ŗH-O-H£®
£¬H2OµÄ½į¹¹Ź½ĪŖ£ŗH-O-H£®
| ||
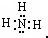 £¬H-O-H
£¬H-O-H
| ||


 Ģį·Ö°Ł·Ö°Ł¼ģ²ā¾ķĻµĮŠ“š°ø
Ģį·Ö°Ł·Ö°Ł¼ģ²ā¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ


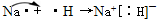
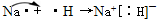




²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
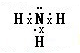
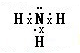
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| 1 |
| 2 |
| 1 |
| 2 |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
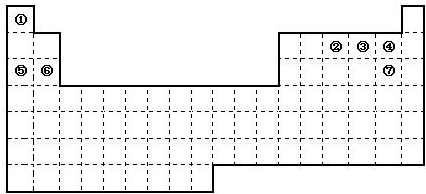
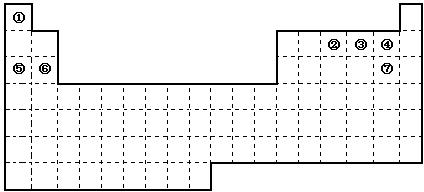
£Ø16·Ö£©Ķ¼ŹĒŌŖĖŲÖÜĘŚ±ķµÄæņ¼Ü£¬ŅĄ¾ŻŌŖĖŲÖÜĘŚ±ķ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ÖÜĘŚ±ķÖŠµÄŌŖĖŲ¢ŻŗĶŌŖĖŲ¢ŽµÄ×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļ¼īŠŌĒæČõĖ³ŠņŹĒ £ØÓĆ»ÆѧŹ½±ķŹ¾£©£¬ÖÜĘŚ±ķÖŠµÄŌŖĖŲ¢ÜŗĶŌŖĖŲ¢ßµÄĒā»ÆĪļµÄ·ŠµćøßµĶĖ³ŠņŹĒ £ØÓĆ»ÆѧŹ½±ķŹ¾£©”£
£Ø2£©¢Ł”«¢ßŌŖĖŲµÄµ„ÖŹ£¬ŌŚ³£ĪĀĻĀ»ÆѧŠŌÖŹĪČ¶Ø£¬Ķس£æÉÓĆ×÷±£»¤ĘųµÄŹĒ £ØĢīŠ“»ÆѧŹ½£©”£
£Ø3£©¢ŁŗĶ¢ŚµÄµ„ÖŹŌŚŅ»¶ØĢõ¼žĻĀ×Ŗ»ÆĪŖ»ÆŗĻĪļA£¬ĒėŠ“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ £¬¼°AµÄ½į¹¹Ź½ ”£
£Ø4£©¢ŚŗĶ¹čŠĪ³ÉµÄ»ÆŗĻĪļŹĒ»Æ¹¤ŠŠŅµŅŃŗĻ³ÉµÄŅ»ÖÖÓ²¶ČŗÜ“ó”¢ČŪµćŗÜøߵľ§Ģ壬ČōŅŃÖŖŌŚ“Ė»ÆŗĻĪļÖŠø÷ŌŖĖŲ¾ł“¦ÓŚĘä×īøß»ņ×īµĶ¼ŪĢ¬£¬¾Ż“ĖĶʶĻ£ŗøĆ»ÆŗĻĪļµÄ»ÆѧŹ½æÉÄÜŹĒ £¬ ÓėøĆ»ÆŗĻĪļ¾§ĢåĄąŠĶĻąĶ¬µÄŹĒ______£ØĒėÓĆĻąÓ¦µÄ±ąŗÅĢīŠ“£©”£
£Ø5£©ĒėŠ“³ö¹¤ŅµÉĻÓƵē½ā±„ŗĶŹ³ŃĪĖ®Öʱø¢ßµ„ÖŹµÄ»Æѧ·½³ĢŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com