
“¼ÓėĒāĀ±Ėį·“Ó¦ŹĒÖʱøĀ±“śĢžµÄÖŲŅŖ·½·Ø”£ŹµŃéŹŅÖʱøäåŅŅĶéµÄ×°ÖĆČēĻĀĶ¼ĖłŹ¾£¬Ęä֊׶ŠĪĘæbŹĒ°²Č«Ę棬ŹŌ¹Üd֊װӊɣĮæÕōĮóĖ®”£ŅŃÖŖäåŅŅĶéµÄ·ŠµćĪŖ38.4”ę£¬ĆܶČĪŖ1.43 g/mL£»
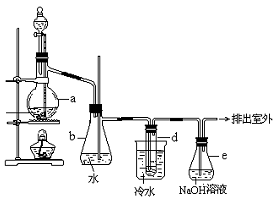
£Ø1£©ÖʱøäåŅŅĶéŠčŅŖÓƵ½ÕōĮóĖ®”¢ŅŅ“¼”¢äå»ÆÄĘ”¢ÅØĮņĖį£¬ŌŚÉÕĘæÖŠ¼ÓČėÕā¼øÖÖĪļÖŹµÄĖ³ŠņŅĄ“ĪŹĒ_____________________________________________________________________”£
£Ø2£©Š”»š¼ÓČČ£¬ŌŚÉÕĘæÖŠ·¢ÉśµÄÖ÷ŅŖ·“Ó¦ÓŠ¢Ł ___________________________________£»
¢Ś__________________________________________________________________________”£
£Ø3£©ĄäĖ®µÄ×÷ÓĆ_______________________£»dŹŌ¹ÜÖŠµÄĻÖĻó_______________________”£
£Ø4£©ÓĆÕāÖÖ·½·ØÖĘČ”µÄäåŅŅĶéÖŠµÄŗ¬ÉŁĮæŌÓÖŹBr2£¬Óū³żČ„äå“śĶéÖŠµÄÉŁĮæŌÓÖŹBr2£¬ĻĀĮŠ¹©Ń”ŹŌ¼ĮÖŠ×īŹŹŗĻµÄŹĒ__________”£
A£®NaIČÜŅŗ ”” B£®NaOHČÜŅŗ ”” C£®Na2SO3ČÜŅŗ ”” D£®KClČÜŅŗ
£Ø5£©ČŻĘ÷eÖŠNaOHČÜŅŗµÄ×÷ÓĆŹĒ_____________________________________________”£
 Ć¢¹ū½ĢøØ“ļ±ź²āŹŌ¾ķĻµĮŠ“š°ø
Ć¢¹ū½ĢøØ“ļ±ź²āŹŌ¾ķĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ±ķÖŠ¶ŌÓŚĻą¹ŲĪļÖŹµÄ·ÖĄąČ«²æÕżČ·µÄŅ»×éŹĒ
| ±ąŗÅ | “æ¾»Īļ | »ģŗĻĪļ | Čõµē½āÖŹ | ·Ēµē½āÖŹ |
| A | Ć÷·Æ | ÕįĢĒ | NaHCO3 | CO2 |
| B | ĢģČ»Ļš½ŗ | ŹÆøą | NH3”¤H2O | CH3CH2OH |
| C | ±ł | ĶõĖ® | H2SiO3 | Cl2 |
| D | µØ·Æ | Ė®²£Į§ | H2CO3 | SO2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
±Č½ĻĻĀĮŠ¾§ĢåµÄĪļĄķŠŌÖŹ
| ½šøÕŹÆ | ¶žŃõ»ÆĢ¼ | ĀČ»ÆÄĘ | ½šŹōĶ | |
| ×é³É¾§ĢåµÄĮ£×Ó | ||||
| ¾§ĢåĮ£×Ó¼äĻą»„×÷ÓĆ | ||||
| ČŪ”¢·Šµć | ||||
| Ó²¶Č | ||||
| µ¼µē | ||||
| µ¼ČČŠŌ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ·“Ó¦µÄ²śĪļÖŠ£¬ÓŠµÄÓŠĶ¬·ÖŅģ¹¹Ģ壬ӊµÄƻӊĶ¬·ÖŅģ¹¹Ģ壬ĘäÖŠŅ»¶Ø²»“ęŌŚĶ¬·ÖŅģ¹¹ĢåµÄ·“Ó¦ŹĒ£Ø £©
A£®ŅģĪģ¶žĻ©£Ø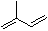 £©ÓėµČĪļÖŹµÄĮæµÄBr2·¢Éś¼Ó³É·“Ó¦
£©ÓėµČĪļÖŹµÄĮæµÄBr2·¢Éś¼Ó³É·“Ó¦
B£®2-Āȶ”Ķé£ØCH3CHClCH2CH3£©ÓėNaOHŅŅ“¼ČÜŅŗ¹²ČČ
C£®¼×±½ŌŚŅ»¶ØĢõ¼žĻĀ·¢ÉśĻõ»ÆÉś³ÉŅ»Ļõ»ł¼×±½µÄ·“Ó¦
D£®¼×“¼ŌŚĶ“ß»ÆŗĶ¼ÓČČĢõ¼žĻĀÉś³ÉµÄ²śĪļ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŃÖŖijӊ»śĪļµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ70£¬ĒŅŗģĶā¹āĘ×±ķÕ÷µ½C=CŗĶC=OµÄ“ęŌŚ£¬1HŗĖ“Ź²ÕńĘ×ČēĻĀĶ¼£Ø·åĆ껿֮±ČŅĄ“ĪĪŖ1:1:1:3£©£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø £©
A£®·Ö×ÓÖŠ¹²ÓŠ5ÖÖ»Æѧ»·¾³²»Ķ¬µÄĒāŌ×Ó
B£®øĆĪļÖŹµÄ·Ö×ÓŹ½ĪŖC4H8O
C£®øĆÓŠ»śĪļµÄ½į¹¹¼ņŹ½ĪŖCH3CH=CHCHO
D£®ŌŚŅ»¶ØĢõ¼žĻĀ£¬1moløĆÓŠ»śĪļæÉÓė3molµÄĒāĘų¼Ó³É
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²ÄĮĻÓė»ÆѧĆÜĒŠĻą¹Ų£¬±ķÖŠ¶ŌÓ¦¹ŲĻµ“ķĪóµÄŹĒ £Ø””””£©”£
| ”” | ²ÄĮĻ | |
| A | øÕÓń”¢½šøÕŹÆ | ČżŃõ»Æ¶žĀĮ |
| B | “óĄķŹÆ”¢ŹÆ»ŅŹÆ | Ģ¼ĖįøĘ |
| C | ĘÕĶØĖ®Äą”¢ĘÕĶز£Į§ | ¹čĖįŃĪ |
| D | ɳ×Ó”¢ŹÆÓ¢ | ¶žŃõ»Æ¹č |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
»ÆѧŠĖȤŠ”×é²éŌÄ׏ĮĻµĆÖŖ£ŗ¼×Č©ŹĒŅ»ÖÖĪŽÉ«ÓŠ“Ģ¼¤ŠŌĘųĪ¶µÄĘųĢ壬·ŠµćĪŖ£19.5 ”ę£¬Ņ×ČÜÓŚĖ®£¬Ņ×±»øßĆĢĖį¼ŲŃõ»ÆÉś³ÉĘäĖūĘųĢ唣¼×Č©¶ŌČĖµÄʤ·ōŗĶŗōĪüĘ÷¹ŁÓŠĒæĮŅµÄ“Ģ¼¤×÷ÓĆ”£“󶹏ż×°ŠŽ²ÄĮĻÖŠŗ¬ÓŠ¼×Č©”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©COŹ¹ČĖÖŠ¶¾ŹĒŅņĪŖŗÜČŻŅ×ŗĶČĖĢåŃŖŅŗÖŠµÄ””””””””½įŗĻ”£
£Ø2£©øĆŠĖȤŠ”×éŹÕ¼ÆV1 Lŗ¬ÓŠ¼×Č©ŗĶCOµÄij¾ÓŹŅæÕĘų£¬ÓĆČēĶ¼×°ÖĆ²ā¶ØĘäÖŠ¼×Č©ŗĶCOµÄŗ¬Į攣Ōņ×°ÖĆAÖŠŹ¢·ÅµÄŹŌ¼ĮŹĒ””””””””£¬ŹµŃéÖŠ»¹ŠčŅŖ²ā¶ØµÄŹż¾ŻŹĒ”””””””””£ĪŖĮĖ»ńµĆ½Ļ×¼Č·µÄŹµŃé½įĀŪ£¬ĖūĆĒŠčŅŖ²ÉČ”µÄ·½·ØŹĒ””””””””£ØĢīŠņŗÅ£©”£
a£®×ŠĻø×öŗĆĆæ²½²Ł×÷£¬Ö»×öŅ»“ĪŹµŃé
b£®ŌŚA”¢B¼ä¼ÓŅ»øöøÉŌļ×°ÖĆ
c£®ÖŲø“×ö2”«3“ĪÉĻŹöŹµŃé
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
»ÆѧŌŚÉś²śŗĶČÕ³£Éś»īÖŠÓŠ×ÅÖŲŅŖµÄÓ¦ÓĆ”£ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ(””””)
A£®Ć÷·ÆĖ®½āŠĪ³ÉµÄAl(OH)3½ŗĢåÄÜĪüø½Ė®ÖŠµÄŠüø”Īļ£¬æÉÓĆÓŚĖ®µÄ¾»»Æ
B£®ŌŚŗ£ĀÖĶāæĒÉĻĻāČėŠææ飬æɼõ»ŗ“¬ĢåµÄøÆŹ“ĖŁĀŹ
C£®MgOµÄČŪµćŗÜøߣ¬æÉÓĆÓŚÖĘ×÷ÄĶøßĪĀ²ÄĮĻ
D£®µē½āMgCl2±„ŗĶČÜŅŗ£¬æÉÖĘµĆ½šŹōĆ¾
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
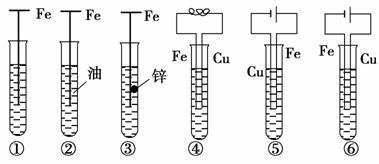
ĻĀĶ¼ÖŠ£¬ĢśøÆŹ“ÓÉæģµ½ĀżµÄĖ³ŠņĪŖ£ØĪ“±ź×¢µÄµē½āÖŹČÜŅŗĪŖĖ®£©_________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com