
��10�֣���1����Һ̬HCl��BaSO4����ˮ����ˮ�����SO2�����ڵ�KCl��Cu��ƾ���Һ�����������ڵ���ʵ��� �ܵ������
����ǿ����ʵ��� ���ڷǵ���ʵ���
��2���ڱ�״����a. 6.72L CH4���� b.3.01��10 23��HCl������� c.13.6g H2S���� d.0.2molNH3�����ж�����������Ĺ�ϵ�Ӵ�С�������ǣ���������ű�ʾ��
23��HCl������� c.13.6g H2S���� d.0.2molNH3�����ж�����������Ĺ�ϵ�Ӵ�С�������ǣ���������ű�ʾ��
��������������ʵ��� ��
�ڱ�״���������������� ��
��������������� ��
��3��������A��һ�ֲ��ȶ������ʣ����ķ�����ɿ���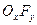 ��ʾ��10mLA�����ֽܷ�����15mL
��ʾ��10mLA�����ֽܷ�����15mL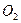 ��10mL
��10mL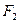 (ͬ�¡�ͬѹ)��
(ͬ�¡�ͬѹ)��
A�Ļ�ѧʽ��___________���ƶ�������___________��
 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��10�֣���1����Һ̬HCl��BaSO4����ˮ����ˮ�����SO2�����ڵ�KCl��Cu��ƾ���Һ�����������ڵ���ʵ��� �ܵ������
����ǿ����ʵ��� ���ڷǵ���ʵ���
��2���ڱ�״����a. 6.72L CH4���� b.3.01��1023��HCl������� c.13.6g H2S���� d.0.2molNH3�����ж�����������Ĺ�ϵ�Ӵ�С�������ǣ���������ű�ʾ��
��������������ʵ��� ��
�ڱ�״���������������� ��
��������������� ��
��3��������A��һ�ֲ��ȶ������ʣ����ķ�����ɿ���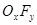 ��ʾ��10mLA�����ֽܷ�����15mL
��ʾ��10mLA�����ֽܷ�����15mL ��10mL
��10mL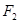 (ͬ�¡�ͬѹ)��
(ͬ�¡�ͬѹ)��
A�Ļ�ѧʽ��___________���ƶ�������___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣���1����Һ̬HCl��BaSO4����ˮ����ˮ�����SO2�����ڵ�KCl��Cu��ƾ���Һ�����������ڵ���ʵ��� �ܵ������
����ǿ����ʵ��� ���ڷǵ���ʵ���
��2���ڱ�״����a. 6.72L CH4���� b.3.01��1023��HCl������� c.13.6g H2S���� d.0.2molNH3�����ж�����������Ĺ�ϵ�Ӵ�С�������ǣ���������ű�ʾ��
��������������ʵ��� ��
�ڱ�״���������������� ��
��������������� ��
��3��������A��һ�ֲ��ȶ������ʣ����ķ�����ɿ���![]() ��ʾ��10mLA�����ֽܷ�����15mL
��ʾ��10mLA�����ֽܷ�����15mL![]() ��10mL
��10mL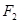 (ͬ�¡�ͬѹ)��
(ͬ�¡�ͬѹ)��
A�Ļ�ѧʽ��___________���ƶ�������___________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com