
���� ���ˡ������õ�14.51g��ɫ����Ϊ���ᱵ��̼�ᱵ����������������ϡ���ᴦ���������������ٵ�4.66g�������ᱵΪ4.66g��̼�ᱵ����Ϊ��14.51g-4.66g=9.85g��
��1�����ݷ�����֪14.51g��ɫ��������ɣ�Ȼ��ֱ�д�����ɳ��������ӷ���ʽ��
��2������n=$\frac{m}{M}$�������ᱵ�����ʵ���������������غ���n��Na2SO4��=n��BaSO4�����ٸ���c=$\frac{n}{V}$����ԭ���Һ��Na2SO4�����ʵ���Ũ�ȣ�
��3������n=$\frac{m}{M}$����̼�ᱵ�����ʵ��������ɵ�����Ϊ������̼������̼Ԫ���غ���n��CO2��=n��BaCO3�����ٸ���V=nVm���������̼�������
��4�����˳�14.51g��������Һ������ΪNaOH�������������غ�n��NaOH��=2n��Na2SO4��+2n��Na2CO3�����ٸ���c=$\frac{n}{V}$����ԭ���Һ��NaOH�����ʵ���Ũ�ȣ�
��� �⣺��1�����ˡ������õ�14.51g��ɫ����Ϊ���ᱵ��̼�ᱵ����������������ϡ���ᴦ���������������ٵ�4.66g�������ᱵΪ4.66g��̼�ᱵ����Ϊ��14.51g-4.66g=9.85g������̼�ᱵ�����ᱵ�����ķ�Ӧ�ֱ�Ϊ��Ba2++CO32-=BaCO3��Ba2++SO42-=BaSO4��
��14.51g��ɫ���������ᱵ��̼�ᱵ�����ɳ��������ӷ���ʽΪBa2++CO32-=BaCO3��Ba2++SO42-=BaSO4��
��2����������������غ�ɵã�n��Na2SO4��=n��BaSO4��=$\frac{4.66g}{233g/mol}$=0.02mol��ԭ���Һ��Na2SO4�����ʵ���Ũ��Ϊ��$\frac{0.02mol}{0.05L}$=0.4mol/L��
��ԭ���Һ��Na2SO4�����ʵ���Ũ��Ϊ0.4mol/L��
��3������CԪ���غ��֪��n��CO2��=n��BaCO3��=$\frac{9.85g}{197g/mol}$=0.05mol�����״�������ɶ�����̼�����Ϊ��V��CO2��=0.05mol��22.4L/mol=1.12L��
�𣺲����������ڱ�״���µ����Ϊ1.12L��
��4�����˳�14.51g��������Һ������ΪNaOH�������������غ��֪��n��NaOH��=2n��Na2SO4��+2n��Na2CO3��=2��0.05mol+2��0.02mol=0.14mol����Ϻ���Һ�����Ϊ0.1L��������Һ��NaOH�����ʵ���Ũ��Ϊ��$\frac{0.14mol}{0.1L}$=1.4mol/L��
�𣺵���Һ���ʵ����ʵ���Ũ��Ϊ1.4mol/L��
���� ���⿼��������йؼ��㣬��Ŀ�Ѷ��еȣ����������Ӧ��ʵ��Ϊ����ؼ���ע�������غ㷨���㣬����������ѧ���ķ�����������ѧ����������


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1mol Cl2�볣��������������Һ��ַ�Ӧʱת�Ƶĵ�����Ϊ2NA | |
| B�� | 1molCu������������巴Ӧת�Ƶĵ�����ΪNA | |
| C�� | �����£�1 L 0.1 mol/L MgCl2��Һ�к�Mg2+��Ϊ0.1NA | |
| D�� | ��״���£�2.24L������������������Ϊ0.1 NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �� | B�� | ���� | C�� | ��ϩ�� | D�� | ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 5��10-6NA��DEHP���� | |
| B�� | ����1.2��10-4NA��̼ԭ�ӵ�DEHP���� | |
| C�� | ����2��10-5NA����ԭ�ӵ�DEHP���� | |
| D�� | ����2.0��10-4NA����ԭ�ӵ�DEHP���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | H2SO4 Na2CO3 NaCl CuO | |
| B�� | H2O Ca��OH��2 HCl Na2SO4 | |
| C�� | H2SO4 NaOH Na2CO3 Fe2O3 | |
| D�� | NaOH H2CO3 NaCl CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ȡ | B�� | ���� | C�� | ��Һ | D�� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
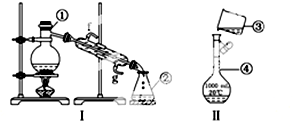
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��ԭ�ԣ�S2-��Cl-��F- | B�� | �ȶ��ԣ�HF��HCl��H2S | ||
| C�� | ���ԣ�KOH��NaOH��Al��OH��3 | D�� | ��ԭ�ԣ�K��Na��Mg |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
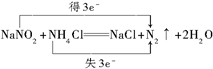 �����������ת�Ƶ���Ŀ�ͷ���
�����������ת�Ƶ���Ŀ�ͷ����鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com