
| ������ | K+��Na+��Fe2+��Ba2+��NH${\;}_{4}^{+}$��Ca2+ |
| ������ | OH-��NO${\;}_{3}^{-}$��I-��HCO${\;}_{3}^{-}$��AlO${\;}_{2}^{-}$��HSO${\;}_{4}^{-}$ |
���� ��1���ٸ��ݡ���Ϻ�ֻ����������ϡ����İ�ɫ��������ʹ��ɫʯ����ֽ���������塱�ƶ�A��B�к�������ӡ������ӡ�����������Ӻ����������ӣ��ٸ���B��ˮ��Һ�ʼ����ж�A��B�����ƣ�
���������������������Ӧ���ȷ�Ӧ�������ᱵ��������ˮ���ݴ�д����Ӧ�����ӷ���ʽ��
��2������Һ��dz��ɫ����Һ�к����������ӣ���ɫ��ӦΪ��ɫ����Һ�к��������ӣ���A��ˮ��Һ�м���ϡ���������������ټ���B����Һ��ƣ�˵�������������·�����������ԭ��Ӧ��A��B����Һһ��������������ӣ���A��ˮ��Һ�м���ϡ��������������˵��A�в��������������ӣ��ٸ������ӹ����ж�A��B����ɣ�
�ڵ����Ӻ��������ӱ����������ɵĵⵥ�ʡ������Ӷ��ܹ�ʹ��Һ��ʾ��ɫ�������ӻ�ԭ��ǿ���������ӣ�
�ۿ���ͨ�����з�Ӧ�����Һ���Ƿ���������ӣ��ж�������Һ��Ƶ�ԭ��
��� �⣺��1����A��B��ˮ��Һ��Ϊ��ɫ��B��ˮ��Һ�ʼ��ԣ��һ�Ϻ�ֻ����������ϡ����İ�ɫ��������ʹ��ɫʯ����ֽ���������壬����Ϊ���ᱵ������Ϊ������˵��A��B�к�����������ӡ������ӡ�����Ӻ����������ӣ�Bˮ��Һ��ʾ���ԣ�B�к������������ӣ��������ӹ��棬BΪBa��OH��2����AΪ������泥�
�ʴ�Ϊ��Ba��OH��2��
��������������������������ʵ���1��1��Ӧ����Ӧ�����Һ��ʾ���ԣ���Ӧ�����ӷ���ʽΪH++SO42-+NH4++Ba2++2OH-$\frac{\underline{\;\;��\;\;}}{\;}$BaSO4��+NH3��+2H2O��
�ʴ�Ϊ��H++SO42-+NH4++Ba2++2OH-$\frac{\underline{\;\;��\;\;}}{\;}$BaSO4��+NH3��+2H2O��
��2����A��ˮ��Һ��dz��ɫ����A��Һ�к���Fe2+��B��ˮ��Һ��ɫ������ɫ��ӦΪ��ɫ����B��Һ�к���Na+����A��ˮ��Һ�м���ϡ���������������ټ���B����Һ��ƣ�˵��A��B��һ��������������ӣ�������A��ˮ��Һ�м���ϡ��������������˵�������������B�У���BΪNaNO3���ܹ������������γɿ����Ե�������I-��HSO4-�����ڡ�A��B��ˮ��Һ����������Ա仯��˵��A��һ�����������ӣ�����AΪFeI2��
�ʴ�Ϊ��FeI2��
��A��Һ�е��������Ӻ͵����Ӷ����л�ԭ�ԣ��ҵ����ӻ�ԭ��ǿ���������ӣ�����������Ӳ��㣬��Һ��Ƶ�ԭ������ӱ������ɵⵥ��ʹ��Һ�ʻ�ɫ�����������ӷ�ӦΪ8H++2NO3-+6I-=2NO��+3I2+4H2O�����������������������������Ӻ���ʣ�࣬�ܹ����������������ӣ�������Һ�ʻ�ɫI-��Fe2+�������������������ӷ�ӦΪ8H++2NO3-+6I-=2NO��+3I2+4H2O��4H++NO3-+3Fe2+=NO��+3Fe3++2H2O��
�ʴ�Ϊ��8H++2NO3-+6I-=2NO��+3I2+4H2O��8H++2NO3-+6I-=2NO��+3I2+4H2O��4H++NO3-+3Fe2+=NO��+3Fe3++2H2O��
������Ӧ�����Һ�д��������ӣ�֤�����������������Ϊ��ȡ���������Һ���Թ��У��μӼ���KSCN��Һ�������������������������
�ʴ�Ϊ��ȡ���������Һ���Թ��У��μӼ���KSCN��Һ�������������������������ɣ���
���� ���⿼���˳������ӵļ��顢δ֪����ƶϡ����ӷ���ʽ��д��֪ʶ������ѧ���ķ�����ʵ�������Ŀ��飬��Ŀ�Ѷ��еȣ������漰�������ϴ�֪ʶ��϶࣬��ֿ�����ѧ������ѧ֪ʶ�����������


 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 ���÷����е�ԭ�ӵ��ӻ���ʽΪsp2��sp3��
���÷����е�ԭ�ӵ��ӻ���ʽΪsp2��sp3���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ��ʼ������pH | ��ȫ������pH | |
| Fe3+ | 1.1 | 3.2 |
| Al3+ | 3.0 | 5.0 |
| Co2+ | 7.2 | 9.2 |
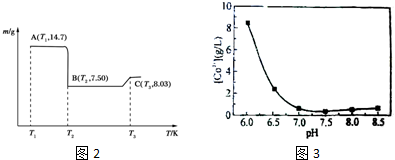
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
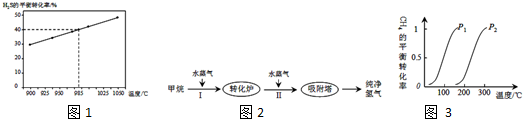
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
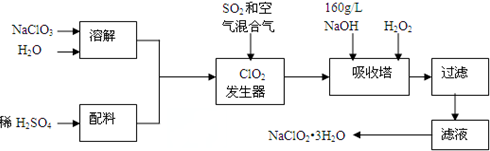
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������Һ������ȩ�е�ȩ����CH3CHO+2Ag��NH3��2++2OH-$\stackrel{ˮԡ����}{��}$CH3COO-+NH4++3NH3+2Ag��+H2O | |
| B�� | ��������Һ��ͨ������CO2��CO2+H2O+2C6H5O-��2C6H5OH+2CO32- | |
| C�� | Na2SO3��Һʹ����KMnO4��Һ��ɫ��5SO32-+4H++2MnO4-�T5SO42-+2Mn2++2H2O | |
| D�� | ��AlCl3��Һ���������ˮ��Al3++4OH-�TAlO2-+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
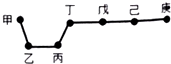
| A�� | ���ʼ��뵥���ҳ�ַ�Ӧһ�����������ɶ��ֻ����� | |
| B�� | ����̬�⻯����ȶ��ԣ����������죾�� | |
| C�� | ����ͨ���ֱ������ڵĽ����Ȼ���ķ���ұ���Һͱ��ĵ��� | |
| D�� | ��Ϊ��Ԫ�صķǽ�������ǿ�����Ը�������������Ӧˮ����������ǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
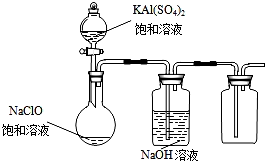 NaClO��KAl��SO4��2������Ҫ�Ļ�����Ʒ������Ӧ������ֽҵ��
NaClO��KAl��SO4��2������Ҫ�Ļ�����Ʒ������Ӧ������ֽҵ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com