

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
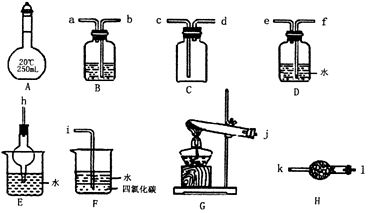
(1)AŅĒĘ÷µÄĆū³ĘŹĒ__________£¬³£ÓĆÓŚ______________________________”£
(2)Óū³żČ„CO2ĘųĢåÖŠ»ģÓŠµÄÉŁĮæSO2£¬æÉŃ”ÓĆ______×°ÖĆ(Ģī×°ÖĆ·ūŗÅ)£¬øĆ×°ÖĆÖŠŹ¢·ÅµÄŹŌ¼ĮæÉŅŌŹĒ______________________________”£
(3)ÓĆĶ¼Ź¾ŅĒĘ÷Éč¼ĘŅ»Ģ×ÖĘČ”“æ¾»”¢øÉŌļµÄ°±ĘųµÄ×°ÖĆ£¬ŅĒĘ÷µÄĮ¬½ÓĖ³ŠņŹĒ(ÓĆ¹ÜæŚ×ÖÄø·ūŗűķŹ¾)j½Ó______________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģ°²»ÕŗĻ·Ź°ĖÖŠøßČżµŚĖÄ“ĪŌĀæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
(12·Ö£©ŌŚÅØ CaCl2ČÜŅŗÖŠĶØČėNH3ŗĶCO2£¬æÉŅŌÖʵĆÄÉĆ×¼¶Ģ¼ĖįøĘ£ØĮ£×ÓÖ±¾¶ŌŚ1~100nmÖ®¼ä£©”£ĻĀĶ¼ĖłŹ¾A~EĪŖŹµŃéŹŅ³£¼ūµÄŅĒĘ÷×°ÖĆ£Ø²æ·Ö¹Ģ¶Ø¼Š³Ö×°ÖĆĀŌČ„£©£¬Ēėøł¾ŻŅŖĒó»Ų“šĪŹĢā”£
CaCl2ČÜŅŗÖŠĶØČėNH3ŗĶCO2£¬æÉŅŌÖʵĆÄÉĆ×¼¶Ģ¼ĖįøĘ£ØĮ£×ÓÖ±¾¶ŌŚ1~100nmÖ®¼ä£©”£ĻĀĶ¼ĖłŹ¾A~EĪŖŹµŃéŹŅ³£¼ūµÄŅĒĘ÷×°ÖĆ£Ø²æ·Ö¹Ģ¶Ø¼Š³Ö×°ÖĆĀŌČ„£©£¬Ēėøł¾ŻŅŖĒó»Ų“šĪŹĢā”£
£Ø1£©ŹµŃéŹŅÖĘČ””¢ŹÕ¼ÆøÉŌļµÄNH3£¬ŠčŃ”ÓĆÉĻŹöŅĒĘ÷×°ÖĆµÄ½ÓæŚĮ¬½ÓĖ³ŠņŹĒ£ØŃ” Ģī×ÖÄø£©£ŗa½Ó________£¬________½Ó________ £¬________½Óh£»
ÓĆA×°ÖĆÖĘČ”NH3µÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ ________________________________________
£Ø2£©ÓĆÓŅĶ¼ĖłŹ¾×°ÖĆŅ²æÉŅŌÖĘČ”NH3£¬ŌņŌ²µ×ÉÕĘæÖŠµÄ¹ĢĢåæÉŅŌŃ”ÓĆ________ £ØŃ”Ģī×ÖÄø±ąŗÅ£©£»
| A£®¼īŹÆ»Ņ | B£®ÉśŹÆ»Ņ | C£®ĪŽĖ®ĀČ»ÆøĘ | D£®ĪŽĖ®ĮņĖįĶ E£®ÉÕ¼ī |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ°²»ÕŹ”øßČżµŚĖÄ“ĪŌĀæ¼»ÆѧŹŌ¾ķ ĢāŠĶ£ŗŹµŃéĢā
(11·Ö£©ŌŚÅØCaCl2ČÜŅŗÖŠĶØČėNH3ŗĶCO2£¬æÉŅŌÖʵĆÄÉĆ×¼¶Ģ¼ĖįøĘ£ØĮ£×ÓÖ±¾¶ŌŚ1~100nmÖ®¼ä£©”£ĻĀĶ¼ĖłŹ¾A~EĪŖŹµŃéŹŅ³£¼ūµÄŅĒĘ÷×°ÖĆ£Ø²æ·Ö¹Ģ¶Ø¼Š³Ö×°ÖĆĀŌČ„£©£¬Ēėøł¾ŻŅŖĒó»Ų“šĪŹĢā”£

£Ø1£©ŹµŃéŹŅÖĘČ””¢ŹÕ¼ÆøÉŌļµÄNH3£¬ŠčŃ”ÓĆÉĻŹöŅĒĘ÷×°ÖĆµÄ½ÓæŚĮ¬½ÓĖ³ŠņŹĒ£ØŃ” Ģī×ÖÄø£©£ŗa½Ó £¬ ½Ó £¬ ½Óh£»
ÓĆA×°ÖĆÖĘČ”NH3µÄ»Æѧ·“Ó¦·½³ĢŹ½ĪŖ
£Ø2£©ÓĆĻĀĶ¼ĖłŹ¾×°ÖĆŅ²æÉŅŌÖĘČ”NH3£¬ŌņŌ²µ×ÉÕĘæÖŠµÄ¹ĢĢåæÉŅŌŃ”ÓĆ £ØŃ”Ģī×ÖÄø±ąŗÅ£©£»

A”¢¼īŹÆ»Ņ B”¢ÉśŹÆ»Ņ C”¢ĪŽĖ®ĀČ»ÆøĘ D”¢ĪŽĖ®ĮņĖįĶ E”¢ÉÕ¼ī
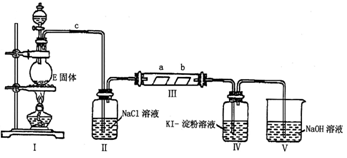
£Ø3£©ĻņÅØCaCl2ČÜŅŗÖŠĶØČėNH3ŗĶCO2ĘųĢåÖĘÄÉĆ×¼¶Ģ¼ĖįøĘ Ź±£¬Ó¦ĻČĶØČėµÄĘųĢåŹĒ £¬ŹŌŠ“³öÖĘÄÉĆ×¼¶Ģ¼ĖįøʵĻÆѧ·½³ĢŹ½ £»
£Ø4£©ŹŌÉč¼Ę¼ņµ„µÄŹµŃé·½°ø£¬ÅŠ¶ĻĖłµĆĢ¼ĖįøĘѳʷæÅĮ£ŹĒ·ńĪŖÄÉĆ×¼¶
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
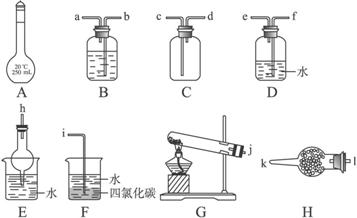
ĻĀĶ¼ĖłŹ¾A~HĪŖŹµŃéŹŅ³£¼ūµÄŅĒĘ÷”¢×°ÖĆ£Ø²æ·Ö¹Ģ¶Ø¼Š³Ö×°ÖĆĀŌČ„£©£¬Ēėøł¾ŻŅŖĒó»Ų“šĻĀĮŠø÷Ģā”£

£Ø1£©AŅĒĘ÷µÄĆū³ĘŹĒ £¬³£ÓĆÓŚ £»
£Ø2£©Ō¤³żČ„CO2ĘųĢåÖŠ»ģÓŠµÄÉŁĮæSO2£¬æÉŃ”ÓĆ ×°ÖĆ£ØĢī×°ÖĆ·ūŗÅ£©£¬øĆ×°ÖĆÖŠŹ¢·ÅµÄŹŌ¼ĮæÉŅŌŹĒ £»
£Ø3£©ÓĆĶ¼Ź¾ŅĒĘ÷Éč¼ĘŅ»Ģ×ÖĘČ”“æ¾»”¢øÉŌļµÄ°±ĘųµÄ×°ÖĆ£¬ŅĒĘ÷µÄĮ¬½ÓĖ³ŠņŹĒ£ØÓĆ¹ÜæŚ×ÖÄø·ūŗűķŹ¾£©j½Ó ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com