
������Ѫ�쵰����Ҫ��ɳɷ֣�������������֯����O2�����ã����ȱ���Ϳ��ܳ���ȱ����ƶѪ�����������������Ҳ�к���������һ�ֳ�����ҩƷ˵�����еIJ������ݣ���ҩƷ��Fe2+33%��36%��������ˮ�������������е�θ���Vc��ά����C��ͬ�������ӱ�Ʒ���ա�
��һ����ͬѧ�����������ʵ����ò���ҩƷ���Ƿ���Fe2+��̽��Vc�����ã�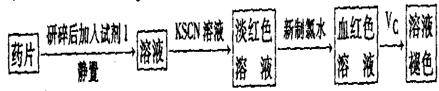
��1������������ˮ����Һ�з��������ӷ�Ӧ����ʽ��_________��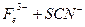

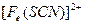 ��
��
��2������KSCN��Һ����Һ��Ϊ����ɫ��˵����Һ��������Fe3+�������Ӵ��ڵ�ԭ������ǣ����ţ�_____________________��
��A��ҩƷ�е���������Ӧ��������������ʽ����
B������ҩ��������������������
C��ҩƷ�������������������������
��3����Ѫ��ɫ��Һ�м���һƬVcƬ��Ƭ�̺���ҺѪ��ɫ��ȥ��˵��Vc��_______�ԣ�ҩƷ˵�����С���Vcͬ�������ӱ�Ʒ���ա���˵������_______________________��
��������ͬѧ�����������������ø�����ر���Һ�ζ��ķ����ⶨ��ҩƷ�Ƿ�ϸ�Ӧԭ��Ϊ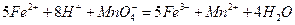 ��ȷ��������ҩƷ10.00g������ȫ�������Լ�2�У����Ƴ�1000mL��Һ��ȡ��20.00mL����0.0200mol/L��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00mL��
��ȷ��������ҩƷ10.00g������ȫ�������Լ�2�У����Ƴ�1000mL��Һ��ȡ��20.00mL����0.0200mol/L��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00mL��
��4����ʵ���е��Լ�2���ͬѧ��Ƶ�ʵ���е��Լ�1��������______������ţ���
A������ˮ B��ϡ���� C��ϡ���� D��ϡ����
��5����ʵ��ζ������в����ζ��ܵ�ͼʾ��ȷ����_______�����ţ���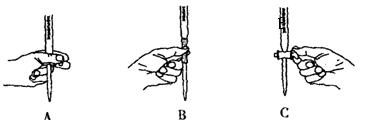
��6����ͨ�����㣬˵����ҩƷ�����������Ƿ�ϸ�д����Ҫ������̣���
 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
������Ѫ�쵰����Ҫ��ɳɷ֣�������������֯����O2�����ã����ȱ���Ϳ��ܳ���ȱ����ƶѪ�����������������Ҳ�к���������һ�ֳ�����ҩƷ˵�����еIJ������ݣ���ҩƷ��Fe2+33%��36%��������ˮ�������������е�θ���Vc��ά����C��ͬ�������ӱ�Ʒ���ա�
��һ����ͬѧ�����������ʵ����ò���ҩƷ���Ƿ���Fe2+��̽��Vc�����ã�
��1������������ˮ����Һ�з��������ӷ�Ӧ����ʽ��_________��
![]()
![]()
![]() ��
��
��2������KSCN��Һ����Һ��Ϊ����ɫ��˵����Һ��������Fe3+�������Ӵ��ڵ�ԭ������ǣ����ţ�_____________________��
��A��ҩƷ�е���������Ӧ��������������ʽ����
B������ҩ��������������������
C��ҩƷ�������������������������
��3����Ѫ��ɫ��Һ�м���һƬVcƬ��Ƭ�̺���ҺѪ��ɫ��ȥ��˵��Vc��_______�ԣ�ҩƷ˵�����С���Vcͬ�������ӱ�Ʒ���ա���˵������_______________________��
��������ͬѧ�����������������ø�����ر���Һ�ζ��ķ����ⶨ��ҩƷ�Ƿ�ϸ�Ӧԭ��Ϊ![]() ��ȷ��������ҩƷ10.00g������ȫ�������Լ�2�У����Ƴ�1000mL��Һ��ȡ��20.00mL����0.0200mol/L��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00mL��
��ȷ��������ҩƷ10.00g������ȫ�������Լ�2�У����Ƴ�1000mL��Һ��ȡ��20.00mL����0.0200mol/L��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00mL��
��4����ʵ���е��Լ�2���ͬѧ��Ƶ�ʵ���е��Լ�1��������______������ţ���
A������ˮ B��ϡ���� C��ϡ���� D��ϡ����
��5����ʵ��ζ������в����ζ��ܵ�ͼʾ��ȷ����_______�����ţ���
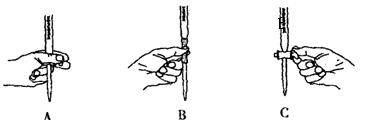
��6����ͨ�����㣬˵����ҩƷ�����������Ƿ�ϸ�д����Ҫ������̣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
������Ѫ�쵰����Ҫ��ɳɷ֣�������������֯����O2�����ã����ȱ���Ϳ��ܳ���ȱ����ƶѪ�����������������Ҳ�к���������һ�ֳ�����ҩƷ˵�����еIJ������ݣ���ҩƷ��Fe2+33%��36%��������ˮ�������������е�θ���Vc��ά����C��ͬ�������ӱ�Ʒ���ա�
��һ����ͬѧ�����������ʵ����ò���ҩƷ���Ƿ���Fe2+��̽��Vc�����ã�
��1������������ˮ����Һ�з��������ӷ�Ӧ����ʽ��_________��
![]()
![]() ��
��
��2������KSCN��Һ����Һ��Ϊ����ɫ��˵����Һ��������Fe3+�������Ӵ��ڵ�ԭ������ǣ����ţ�_____________________��
��A��ҩƷ�е���������Ӧ��������������ʽ����
B������ҩ��������������������
C��ҩƷ�������������������������
��3����Ѫ��ɫ��Һ�м���һƬVcƬ��Ƭ�̺���ҺѪ��ɫ��ȥ��˵��Vc��_______�ԣ�ҩƷ˵�����С���Vcͬ�������ӱ�Ʒ���ա���˵������_______________________��
��������ͬѧ�����������������ø�����ر���Һ�ζ��ķ����ⶨ��ҩƷ�Ƿ�ϸ�Ӧԭ��Ϊ![]() ��ȷ��������ҩƷ10.00g������ȫ�������Լ�2�У����Ƴ�1000mL��Һ��ȡ��20.00mL����0.0200mol/L��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00mL��
��ȷ��������ҩƷ10.00g������ȫ�������Լ�2�У����Ƴ�1000mL��Һ��ȡ��20.00mL����0.0200mol/L��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00mL��
��4����ʵ���е��Լ�2���ͬѧ��Ƶ�ʵ���е��Լ�1��������______������ţ���
A������ˮ B��ϡ���� C��ϡ���� D��ϡ����
��5����ʵ��ζ������в����ζ��ܵ�ͼʾ��ȷ����_______�����ţ���
��6����ͨ�����㣬˵����ҩƷ�����������Ƿ�ϸ�д����Ҫ������̣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�߰���ѧ�߶���ѧ�ڵ����ζο���ѧ�Ծ� ���ͣ�ʵ����
������Ѫ�쵰����Ҫ��ɳɷ֣�������������֯����O2�����ã����ȱ���Ϳ��ܳ���ȱ����ƶѪ�����������������Ҳ�к���������һ�ֳ�����ҩƷ˵�����еIJ������ݣ���ҩƷ��Fe2+33%��36%��������ˮ�������������е�θ���Vc��ά����C��ͬ�������ӱ�Ʒ���ա�
��һ����ͬѧ�����������ʵ����ò���ҩƷ���Ƿ���Fe2+��̽��Vc�����ã�
��1������������ˮ����Һ�з��������ӷ�Ӧ����ʽ��_________��
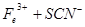
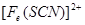 ��
��
��2������KSCN��Һ����Һ��Ϊ����ɫ��˵����Һ��������Fe3+�������Ӵ��ڵ�ԭ������ǣ����ţ�_____________________��
��A��ҩƷ�е���������Ӧ��������������ʽ����
B������ҩ��������������������
C��ҩƷ�������������������������
��3����Ѫ��ɫ��Һ�м���һƬVcƬ��Ƭ�̺���ҺѪ��ɫ��ȥ��˵��Vc��_______�ԣ�ҩƷ˵�����С���Vcͬ�������ӱ�Ʒ���ա���˵������_______________________��
��������ͬѧ�����������������ø�����ر���Һ�ζ��ķ����ⶨ��ҩƷ�Ƿ�ϸ�Ӧԭ��Ϊ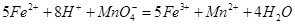 ��ȷ��������ҩƷ10.00g������ȫ�������Լ�2�У����Ƴ�1000mL��Һ��ȡ��20.00mL����0.0200mol/L��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00mL��
��ȷ��������ҩƷ10.00g������ȫ�������Լ�2�У����Ƴ�1000mL��Һ��ȡ��20.00mL����0.0200mol/L��KMnO4��Һ�ζ�����ȥKMnO4��Һ12.00mL��
��4����ʵ���е��Լ�2���ͬѧ��Ƶ�ʵ���е��Լ�1��������______������ţ���
A������ˮ B��ϡ���� C��ϡ���� D��ϡ����
��5����ʵ��ζ������в����ζ��ܵ�ͼʾ��ȷ����_______�����ţ���
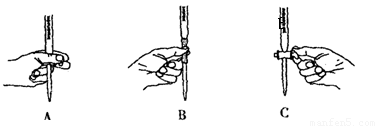
��6����ͨ�����㣬˵����ҩƷ�����������Ƿ�ϸ�д����Ҫ������̣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ѫ�쵰����Ҫ��ɳɷ֣�������������֯����O2 �����ã���ȱ���Ϳ��ܳ���ȱ����ƶѪ��������һ�ֳ�������ҩƷ˵�����еIJ������ݣ���ҩƷ��Fe2+ 33����36����������ˮ�������������е�θ���Vc��ά����C��ͬ�������ӱ�Ʒ���ա�
��.��ͬѧ���������ʵ����ò���ҩƷ���Ƿ���Fe2+��̽��Vc�����ã�
![]()
�ż���������ˮ����Һ�з��������ӷ�Ӧ����ʽ��_____________�� ��
�Ƽ���KSCN����Һ�䵭��ɫ��˵����Һ��������Fe3+�������Ӵ��ڵĿ���ԭ��___________��
A��ҩƷ�е���������Ӧ��������������ʽ����
B����ʵ������������������������� C��ҩƷ�������������������������
����Ѫ��ɫ��Һ�м���һƬVcƬ��Ƭ�̺���ҺѪ��ɫ��ȥ��˵��Vc�� �ԡ�
��.��ͬѧ�������Ը�����ر�Һ�ζ����ⶨ��ҩƷ�Ƿ�ϸ�ԭ����5Fe2++8H++MnO4-��5Fe3++2Mn2++4H2O ��ȷ��������ҩƷ10��00g������ȫ�������Լ�2�����1000mL��Һ��ȡ��20��00mL����0��0200 mol��L��1��KMnO4 ��Һ�ζ�����ȥKMnO4��Һ12��00 mL��
�ȸ�ʵ���е��Լ�2���ͬѧ���ʵ���е��Լ�1�������� �����ţ���
A������ˮ B��ϡ���� C��ϡ���� D��ϡ����
�ɱ�ʵ��ζ������в����ζ��ܵ�ͼʾ��ȷ���� �����ţ���
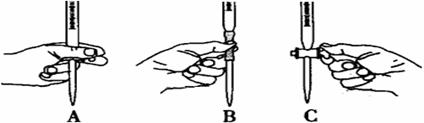
���ж�ʵ�鵽��ζ��յ������Ϊ ��
��7���������ҩƷ����Ԫ�صİٷֺ���Ϊ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com