
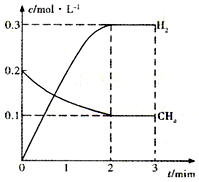
��һ�������£������Ϊ2 L�������м���2 mol O2��3 mol SO2���п��淴Ӧ��2SO2��g��+ O2��g��![]() 2SO3��g����2 min����O2�����ʵ���Ϊ1.6 mol����
2SO3��g����2 min����O2�����ʵ���Ϊ1.6 mol����
��1��2 min�ڣ�SO2�����ʵ��������� mol��SO3�����ʵ��������� mol��
��2������O2��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ���ʣ���V��O2���� ��
��3������SO3��Ũ�ȱ仯����ʾ�÷�Ӧ�ķ�Ӧ���ʣ���V��SO3���� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 2SO3��l����2min����O2�����ʵ���Ϊ1.6mol����
2SO3��l����2min����O2�����ʵ���Ϊ1.6mol�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��ҵ��������Ȼ������Ҫ�ɷ���CH4����H2O���и��������Ʊ��ϳ�����CO+H2����CO��H2��һ�����������Ʊ���ȩ�����ѵȶ����л����֪CH4��H2��CO��ȼ���ȣ���H���ֱ�Ϊ-890.3kJ?mol-1��-285.8kJ?mol-1��-283.0kJ?mol-1��18.0gˮ����Һ��ʱ�������仯Ϊ44.0kJ��
��ҵ��������Ȼ������Ҫ�ɷ���CH4����H2O���и��������Ʊ��ϳ�����CO+H2����CO��H2��һ�����������Ʊ���ȩ�����ѵȶ����л����֪CH4��H2��CO��ȼ���ȣ���H���ֱ�Ϊ-890.3kJ?mol-1��-285.8kJ?mol-1��-283.0kJ?mol-1��18.0gˮ����Һ��ʱ�������仯Ϊ44.0kJ��| t/min | n��CH4��/mol | n��H2O��/mol | n��CO��/mol | n��H2��/mol |
| 4 | 0.18 | 0.38 | 0.22 | 0.66 |
| n(CH4) |
| n(H2O) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 2SO3��2minʱ�����O2�����ʵ���Ϊ1.6mol����
2SO3��2minʱ�����O2�����ʵ���Ϊ1.6mol���� 2SO3��g����H=-2.5QkJ?mol-1
2SO3��g����H=-2.5QkJ?mol-1 2SO3��g����H=-2.5QkJ?mol-1
2SO3��g����H=-2.5QkJ?mol-1�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ҵ���Ʊ��ϳ����Ĺ�����Ҫ��ˮ�����������飺
��ҵ���Ʊ��ϳ����Ĺ�����Ҫ��ˮ�����������飺| A����ƽ��ʱ��CH4��g����ת����Ϊ75% | B��O-10 min �ڣ�v��CO��=0.075 mol?L-1��min-1 | C���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=O.1875 mol?L-1 | D����CH4��g��������������H20��g��������������ȣ���Ӧ����ƽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ��ׯ�и���3��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ҵ���Ʊ��ϳ����Ĺ�����Ҫ��ˮ�����������飺CH4(g) +H2O(g)��CO(g) +3H2(g) ��H��0����һ�������£������Ϊ1L���ܱ������г���1 mol CH4 (g)��1mol H2O(g)�����H2O(g)��H2(g)��Ũ����ʱ��仯 ������ͼ��ʾ������˵����ȷ����
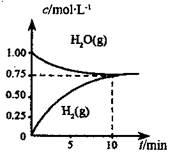
A.��ƽ��ʱ,CH4( g)��ת����Ϊ75%
B.0��10 min �ڣ�v(CO)��0.075 mol•L��1��min-1
C.�÷�Ӧ�Ļ�ѧƽ�ⳣ��K��0.1875 mol•L��1
D.��CH4(g)������������H2O(g)������������ȣ���Ӧ����ƽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com