
������һ�������Դ����������ȡ������������Դ������о��ȵ㣮
��֪��CH4(g)��H2O(g)![]() CO(g)��3H2(g)����H��206.2 kJ��mol��1
CO(g)��3H2(g)����H��206.2 kJ��mol��1
CH4(g)��CO2(g)![]() 2CO(g)��2H2(g)����H��247.4 kJ��mol��1
2CO(g)��2H2(g)����H��247.4 kJ��mol��1
(1)�Լ���Ϊԭ����ȡ�����ǹ�ҵ�ϳ��õ����ⷽ����CH4(g)��H2O(g)��Ӧ����CO2(g)��H2(g)���Ȼ�ѧ����ʽΪ________��
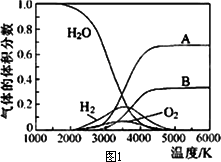
(2)H2O���ȷֽ�Ҳ�ɵõ�H2��������ˮ�ֽ���ϵ����Ҫ���������������¶ȵĹ�ϵ��ͼ1��ʾ��ͼ��A��B��ʾ������������________��
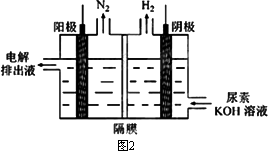
(3)�������[CO(NH2)2]�ļ�����Һ�����װ��ʾ��ͼ��ͼ2(�����и�Ĥ����ֹ����ͨ��������������Ϊ���Ե缫)�����ʱ�������ĵ缫��ӦʽΪ________��
(4)���������Ƶõ�H2���Ժ�CO��һ�������ºϳɼ״��Ͷ�����(CH3OCH3)�������������ʣ������������ʵ���1��1����Ӧ����ԭ�������ʴ�100�����ϳɵ����ʿ�����________��
a������
b���״�
c����ȩ
d������
(5)��һ���¶Ⱥ�ѹǿ�£�CO��H2���ϳɶ����ѵķ�ӦΪ��
3H2(g)��3CO(g)![]() CH3OCH3(g)��CO2(g)
CH3OCH3(g)��CO2(g)
��һ����ɱ���ܱ������г���3 mol��H2��3 mol��CO��1 mol��CH3OCH3��1 mol��CO2����һ��ʱ��ﵽƽ�⣬�����ƽ��ʱ��������ܶ���ͬ��ͬѹ����ʼʱ��1.6������Ӧ��ʼʱ�����淴Ӧ���ʵĴ�С��v(��)________v(��)(�����������������)��������________��ƽ��ʱn(CH3OCH3)��________mol��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������һ�������Դ������ͨ�����ַ����Ƶã�
������һ�������Դ������ͨ�����ַ����Ƶã�| c(CO)c(H2) |
| c(H2O) |
| c(CO)c(H2) |
| c(H2O) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㣮
������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㣮
������һ�������Դ����������ȡ�봢��������Դ����������о��ȵ㣮| 1 |
| 2 |
c(H2)c
| ||
| c(H2O) |
c(H2)c
| ||
| c(H2O) |
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com