
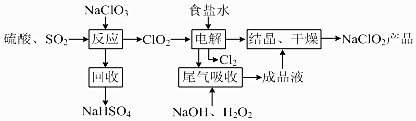
���� ���Ʊ����̿�֪��NaClO3��SO2��H2SO4�ữ����������ClO2������NaClO3�������������ղ���ΪNaHSO4��˵�������������ƣ��Ҳ���ClO2�����ݵ����غ��֪���˷�Ӧ�Ļ�ѧ����ʽΪ2NaClO3+SO2+H2SO4=2NaHSO4+2ClO2��Ȼ����װ��������ClO2�õ�������ClO2-������Cl-ʧ��������Cl2�����������������������Һ����ClO2������ΪClO2-�����NaClO2��Һ�ᾧ������õ���Ʒ���Դ������
��� �⣺��1��NaClO3��SO2��H2SO4�ữ����������ClO2������NaClO2������������ԭ����ΪNaCl�����ղ���ΪNaHSO4��˵�������������ƣ��Ҳ���ClO2�����ݵ����غ��֪���˷�Ӧ�Ļ�ѧ����ʽΪ��2NaClO3+SO2+H2SO4=2NaHSO4+2ClO2��
�ʴ�Ϊ��2NaClO3+SO2+H2SO4=2NaHSO4+2ClO2��
��2������β�����չ����У��������������������Һ����ClO2������ΪClO2-����H2O2Ϊ��ԭ�����ֲ��������µ����ʣ����Կɴ���H2O2���Լ���Na2O2����ѡ��A��
�����β��������Ч�ʣ�����Խ��衢����β����ͨ�����ʡ���������ҺŨ�ȣ���ClO2�ķе�Ϊ283K�����Խ��¶ȿ�����20������Ҳ�������β��������Ч�ʣ���ѡ��AC��
����ͼ��֪�����ú��������������������Һ����ClO2������ΪClO2-����˷�Ӧ��ClO2Ϊ����������ԭ����ΪClO2-�����ϼ۴�+4�۽�Ϊ+3�ۣ�H2O2Ϊ��ԭ������������ΪO2��ÿĦ��H2O2�õ�2mol���ӣ����ݵ����غ��֪�������ͻ�ԭ�������ʵ���֮��Ϊ2��1��
�ʴ�Ϊ��2��1��
��3��HClO2���ȶ����ֽ����Cl2��ClO2��H2O�����ݵ����غ��֪���ֽ�Ļ�ѧ����ʽΪ8HClO2=Cl2��+6ClO2��+4H2O���ʴ�Ϊ��8HClO2=Cl2��+6ClO2��+4H2O��
��4�����Ʊ����̿�֪�����������ΪClO2�Ͷ���SO2���壬�������ú��������������������Һ����β��ClO2�Ͷ���SO2���壬����ΪClO2-����������Na2SO4���������ᾧ���������Һ���˺�NaClO2������NaCl��Na2SO4����NaCl��Na2SO4 ���ܽ�����¶����߶���������38������60�����»�����NaClO2ʱ���ܻ��е�������NaCl��Na2SO4���ʴ�Ϊ��NaCl��Na2SO4��
���� ���⿼�����ʵ��Ʊ�ʵ�飬Ϊ�߿��������ͣ������Ʊ����̼������ķ�ӦΪ���Ĺؼ���������ѧ���ķ���������ʵ�������Ŀ��飬ע��������롢Ԫ�ػ�����֪ʶ�ȣ���Ŀ�ѶȲ���


 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ۢ� | B�� | �٢ڢ� | C�� | �ڢ� | D�� | �٢ۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | С�մ�--Na2CO3 | B�� | ��ʯ��--Ca ��OH��2 | C�� | ��ȩ--CH3OH | D�� | �ռ�--NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������Ȼ�̼����ˮ�е�����ȡ���������÷ֲ���²�Һ��Ϊ��ɫ | |
| B�� | ��ȡ��Һʱ���ӷ�Һ©���¿������²�Һ�壬��ʱ�رգ��ٴ������ϲ�Һ�� | |
| C�� | �������ھƾ�������ˮ���ʿ����þƾ���ȡ��ˮ�еĵ� | |
| D�� | ��ȡ֮��һ��ͨ����Һ©�����������ܵ�Һ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ��װ�ü��ռ�SO2 | |
| B�� | ��װ�����Ʊ�AlCl3���� | |
| C�� | ��װ�ñ������к͵ζ�ʱ���ζ�ǰ��ƿ���ô�װҺ��ϴ | |
| D�� | װ�ö���ʹ�÷�Һ©��������ƿʱ����Ҫ��������Ƿ�©Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | Na+��Mg2+��Cl-��SO42- | B�� | Mg2+��Ca2+��HCO3-��Cl- | ||
| C�� | Ba2+��Al3+��Cl-��NO3- | D�� | K+��Cu2+��Cl-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �������Ժʿ����Ժʿѧ��ͬ���ġ����ӹ��������ն�����������ˣ�ȫ������������Ϸ˵��BF3��TiO2��HCHO��N2O����ï����NH3��HCN��H2S��O3�������ϩ���Ƶ��ڶࡰ���ӹ������е����ǣ�
�������Ժʿ����Ժʿѧ��ͬ���ġ����ӹ��������ն�����������ˣ�ȫ������������Ϸ˵��BF3��TiO2��HCHO��N2O����ï����NH3��HCN��H2S��O3�������ϩ���Ƶ��ڶࡰ���ӹ������е����ǣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�켪��ʡ�����ϵڶ���ģ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
N2O����ҽѧ�ϵ�һ��������������һ�ֳ������������塣��ȡN2O�ķ����кܶ࣬����
��NH4NO3===N2O����2H2O
��K2SO3��2NO===K2SO4��N2O��
��2NH3��2O2===N2O����3H2O
��2NaNO3����NH4��2SO4===2N2O����Na2SO4��4H2O�ȡ�����˵����ȷ����
A����Ӧ��������識������������ǻ�ԭ����H2O����������
B����Ӧ����K2SO3�ǻ�ԭ����N2O����������
C����Ӧ����ÿ����1 mol N2O��ת��8 mol����
D����Ӧ����NaNO3�ǻ�ԭ����N2O���������������ǻ�ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ������ʡ��һ9���¿���ѧ���������棩 ���ͣ������
��1����ҩ���й��ġ��Ĵ�����֮һ����Զֵ�������セ����Ҳ��Զ�ἤ��������ȥ�ܷ�ͼǿ���ڻ�ҩ�ڷ�����ըʱ���������µķ�Ӧ��2KNO3+C��S===K2S+2NO2��+CO2������������Ԫ���� ���������� ����ԭ������ ������ת�Ƶ�����Ϊ__________��
��2��������������2000��ǰ�;�����ʹ���ȶ�����Ч�����Ķ�������ȡ��������������ˮ����֪������������ˮʱ������������ӣ�ClO2-�����ɣ������������뻹ԭ��������ʵ���֮��Ϊ1��1��������������ˮ�ķ�Ӧ����ʽ2ClO2 + H2O��HClO3 + HClO2�����õ����ű�������ת�Ʒ������Ŀ��ָ���������2ClO2 + H2O��HClO3 + HClO2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com