
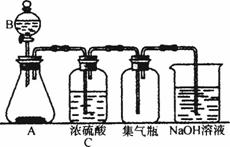
Ķ¼9-4
(1)ĒėÄć·ÖĪöÓĆøĆ×°ÖĆÖĘČ””¢øÉŌļ”¢ŹÕ¼ÆĘųĢåŗĶĪ²ĘųĪüŹÕĘųĢå±ŲŠė·ūŗĻµÄŅŖĒó£ŗ
¢Ł¶Ō·“Ó¦ĪļµÄŅŖĒó£ŗ_____________________________________________________£»
¢Ś¶Ō·“Ó¦Ģõ¼žµÄŅŖĒó£ŗ_____________________________________________________£»
¢Ū¶ŌÉś³ÉĘųĢåŠŌÖŹµÄŅŖĒó£ŗ__________________________________________________”£
(2)ČōÓĆøĆ×°ÖĆÖĘČ”H2SŹ±£¬AÖŠŹĒFeS2”¢BÖŠŹĒĻ”ĮņĖį£¬·“Ó¦ŗóČÜŅŗÖŠÓŠµ»ĘÉ«µÄ»ģ×Ē”£Ōņ£ŗ
¢ŁĶ¼9-4ĖłŹ¾×°ÖĆÓ¦×÷³öµÄ±ä¶ÆÓŠ__________________________________£»
¢ŚAÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ__________________________________£»
¢ŪNaOHČÜŅŗÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ__________________________________”£
 ŠÄĖćæŚĖćĒÉĖćŅ»æĪŅ»Į·ĻµĮŠ“š°ø
ŠÄĖćæŚĖćĒÉĖćŅ»æĪŅ»Į·ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
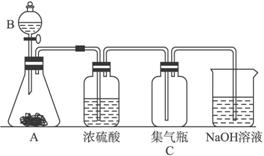
£Ø1£©Ēė·ÖĪöÄÜÓĆøĆ×°ÖĆÖĘČ””¢øÉŌļ”¢ŹÕ¼ÆŗĶĪüŹÕµÄĘųĢå¶Ō·“Ó¦Īļ”¢·“Ó¦Ģõ¼ž¼°ĘųĢåŠŌÖŹ±ŲŠė·ūŗĻµÄŅŖĒó£ŗ
¢Ł¶Ō·“Ó¦ĪļµÄŅŖĒó£ŗ______________________________________________________£»
¢Ś¶Ō·“Ó¦Ģõ¼žµÄŅŖĒó£ŗ____________________________________________________£»
¢Ū¶ŌÉś³ÉĘųĢåŠŌÖŹµÄŅŖĒó£ŗ_________________________________________________”£
£Ø2£©ÓĆøĆ×°ÖĆÖĘČ”ĀČĘųŹ±£¬AÖŠŹĒøßĆĢĖį¼Ų£¬BÖŠŹĒÅØŃĪĖį£¬·“Ó¦ŗóČÜŅŗÖŠ“ęŌŚ“óĮæMn2+”£·Ö±šŠ“³öAÖŠŗĶNaOHČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ
AÖŠ_________________________£¬NaOHČÜŅŗÖŠ______________________________”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com