
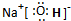 ����I�Ǽ��Է��ӣ���Ի�Ǽ��ԣ�
����I�Ǽ��Է��ӣ���Ի�Ǽ��ԣ�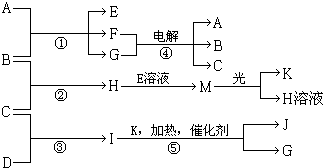
���� I�ķ�����4��ԭ����ɲ�������10�����ӣ�ӦΪNH3��G�ڳ���������ɫҺ�壬ӦΪH2O����ת����ϵ��֪��Ӧ��ӦΪ�����Ĵ���������KΪO��JΪNO������A��E��F����ɫ��Ӧ�ʻ�ɫ��Ӧ��������Ԫ�أ�M�ڹ��������¿�����O2��ӦΪHClO����HΪHCl�����ת����ϵ��֪��BΪCl2��CΪH2��DΪN2��EΪNaClO��AΪNaOH��FΪNaCl���ݴ˽��
��� �⣺I�ķ�����4��ԭ����ɲ�������10�����ӣ�ӦΪNH3��G�ڳ���������ɫҺ�壬ӦΪH2O����ת����ϵ��֪��Ӧ��ӦΪ�����Ĵ���������KΪO��JΪNO������A��E��F����ɫ��Ӧ�ʻ�ɫ��Ӧ��������Ԫ�أ�M�ڹ��������¿�����O2��ӦΪHClO����HΪHCl�����ת����ϵ��֪��BΪCl2��CΪH2��DΪN2��EΪNaClO��AΪNaOH��FΪNaCl��
��1�������Ϸ�����֪BΪCl2��MΪHClO���ʴ�Ϊ��Cl2��HClO��
��2��AΪNaOH�������ʽΪ��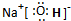 ��IΪNH3���Ǽ��Է��ӣ�
��IΪNH3���Ǽ��Է��ӣ�
�ʴ�Ϊ��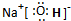 �����ԣ�
�����ԣ�
��3��0.1mol/LNaClOˮ��Һ��ClO-ˮ�⣬��Һ�ʼ��ԣ�������Ũ���ɴ�С��˳��Ϊ��c��Na+����c��ClO-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��ClO-����c��OH-����c��H+����
��4����Ӧ��Ϊ�������������Ƶķ�Ӧ����Ӧ�����ӷ���ʽΪ��Cl2+2OH-=Cl-+ClO-+H2O��
��Ӧ��Ϊ�����Ĵ�������Ӧ����Ӧ�Ļ�ѧ����ʽΪ��4NH3+5O2 $\frac{\underline{����}}{��}$4NO+6H2O��
�ʴ�Ϊ��Cl2+2OH-=Cl-+ClO-+H2O��4NH3+5O2 $\frac{\underline{����}}{��}$4NO+6H2O��
��5����Ӧ������������ԭ��Ӧ��ˮ�������ŵ��������������������缫��ӦʽΪ��2H2O+2e-=H2��+2 OH-����ת��0.02mol���ӣ����ݵ缫��Ӧʽ��֪OH-�����ʵ���Ϊ0.02mol����Һ�������200mL�����ʱ��ҺOH-�����ʵ���Ũ��Ϊ0.1nol/L��������Һ��PH=13��
�ʴ�Ϊ��13��
���� ������Ҫ���鳣��Ԫ�ؼ�����Ҫ����������ʣ��漰����ʽ������Ũ�ȴ�С�Ƚϡ���Ӧ����ʽ����ҺpH�ļ����֪ʶ���е��Ѷȣ����ʵ��ƶ��ǽ��� �Ĺؼ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ϡ�����������������Һ�У�H++OH-�TH2O | |
| B�� | ͭƬ�ϵμ�ϡ���3Cu+8H++2 NO3-�T3Cu2++2NO��+4H2O | |
| C�� | ʯ��ʯͶ�뵽ϡ�����У�CO32-+2 H+�TCO2��+H2O | |
| D�� | Fe�ۼ���ϡ�����У�Fe+2H+�TFe2++H2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ͨ��Ʒ����Һ����Ʒ����Һ��ɫ����˵������CO2���� | |
| B�� | ͨ������ʯ��ˮ��������ǣ���˵������CO2���� | |
| C�� | ��ͨ������NaOH��Һ����ͨ������ʯ��ˮ��������ǣ���˵������CO2���� | |
| D�� | ��ͨ������KMnO4��Һ������ǿ�����ԣ�����ͨ������ʯ��ˮ��������ǣ���˵������CO2���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
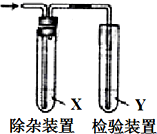 ����ͼ��ʾװ�ü�������ʱ�Լ�Xû��Ҫ���Ǵ�����ǣ�������
����ͼ��ʾװ�ü�������ʱ�Լ�Xû��Ҫ���Ǵ�����ǣ�������| ��������� | �Լ�X | �Լ�Y | |
| A | CH3CH2Br��NaOH�Ҵ���Һ�����Ʊ�����ϩ | H2O | Br2��CCl4��Һ |
| B | CH3CH2OH��ŨH2SO4����170���Ʊ�����ϩ | NaOH��Һ | ����KMnO4��Һ |
| C | ��ʯ�뱥��ʳ��ˮ��Ӧ�Ʊ�����ϩ | ˮ | ����KMnO4��Һ |
| D | ����Һ�壬��м��Ϸ�Ӧ���ɵ�HBr | CCl4 | ��������Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SiO2��NaCl��CO2 | B�� | HF��HCl��HBr | C�� | NaCl��KCl��RbCl | D�� | Na��Mg��Al |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ȩ | B�� | ���� | C�� | �״� | D�� | �ױ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ʴ���ǽ���ԭ��ʧȥ���ӱ���ԭ�Ĺ��� | |
| B�� | ��п��Ƥ�ĶƲ����������ܵ����������Ը�ʴ | |
| C�� | �����ĸ�ʴͨ����������ʴ�����ⸯʴ��ͨ���������������ʴΪ�� | |
| D�� | �ȼҵ�У����������ķ�ӦΪ��2H++2e-�TH2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
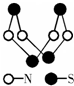
| A�� | �����ʵķ���ʽΪSN | |
| B�� | �����ʵķ�����ֻ�м��Լ���û�зǼ��Լ� | |
| C�� | �����ʷ�����N-S�����ܴܺ��侧���кܴ��Ӳ�� | |
| D�� | �������뻯����S2N2��Ϊͬ�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com