
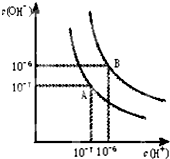
 ��1�������£������0.1mol NH4Cl��0.05mol NaOHȫ������ˮ���γɻ����Һ����������ʧ����
��1�������£������0.1mol NH4Cl��0.05mol NaOHȫ������ˮ���γɻ����Һ����������ʧ����| 10-12 |
| 10-8 |
| 10-12 |
| 10-7 |
| 10-4mol/L��xL-10-5mol/L��yL |
| (x+y)L |
| 10-12 |
| 10-8 |
| 10-4mol/L��x-10-5mol/L��y |
| x+y |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� | H2��g��+O2��g���TH2O��g����H�T-242kJ/mol�� |
| �� | 2H2��g��+O2��g���T2H2O��l����H�T-572kJ/mol�� |
| �� | C��s��+O2��g���TCO��g����H�T-110.5kJ/moL�� |
| �� | C��s��+O2��g���TCO2��g����H�T-393.5kJ/moL�� |
| �� | CO2��g��+2H2O��g���TCH4��g��+2O2��g����H�T+802kJ/moL |
| ��ѧ�� | O=O | C-C | H-H | O-O | C-O | O-H | C-H |
| ����kJ/mol | 497 | 348 | 436 | 142 | 351 | 463 | 414 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
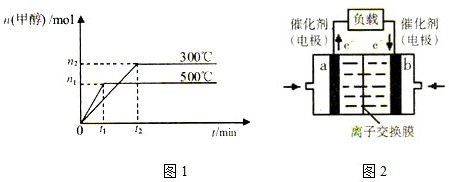
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
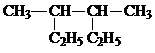 ���ƣ�
���ƣ�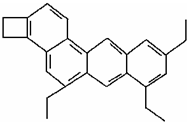
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �����Լ� | ��������ʵĻ�ѧʽ | ���ӷ���ʽ |
| ˮ |
| �����Լ� | ��������ʵĻ�ѧʽ | ���ӷ���ʽ |
| �����Լ� | ��������ʵĻ�ѧʽ | ���ӷ���ʽ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com